
| Tracks 1-17 |
| Track 1 |
Introduction |
| Track 2 |
Importance of waiting for
definitive research data
before using an unproven
treatment approach |
| Track 3 |
Acceptance of trastuzumab
as adjuvant therapy in the
United Kingdom |
| Track 4 |
Biologic rationale for topoisomerase
II-alpha (TOPO II) amplification predicting sensitivity to
anthracyclines |
| Track 5 |
TOPO II amplification and the
efficacy of anthracycline-based
chemotherapy in BCIRG 006 |
| Track 6 |
Clinical use of TOPO II amplification
to select adjuvant
chemotherapy for patients with
HER2-positive disease |
| Track 7 |
Lack of reliable HER2 testing in
the United States |
| Track 8 |
Potential benefit of adjuvant
trastuzumab monotherapy |
|
| Track 9 |
Concurrent versus sequential
administration of trastuzumab
and chemotherapy |
| Track 10 |
Sequential use of trastuzumab to
abrogate cardiac toxicity |
| Track 11 |
Duration of adjuvant trastuzumab |
| Track 12 |
Necessity of clinical trials
examining the duration of
adjuvant trastuzumab |
| Track 13 |
Clinical use of adjuvant trastuzumab
monotherapy |
| Track 14 |
Role of cardiac monitoring in
treatment decision-making
regarding adjuvant trastuzumab |
| Track 15 |
Use of Oncotype DX™ to
determine therapy for patients
with ER-positive, HER2-positive
disease |
| Track 16 |
Quality control in ER and
HER2 testing |
| Track 17 |
Efforts to improve national
standards for ER and HER2
testing |
|
|
Select Excerpts from the Discussion
 Tracks 4-7
Tracks 4-7
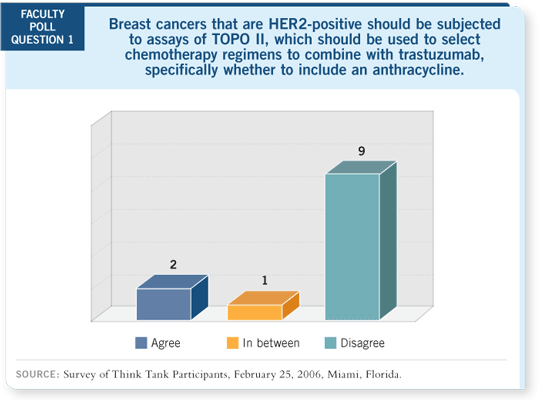
 DR LOVE: Mark, what’s your reaction to the TOPO II data that were
presented as a part of the BCIRG 006 analysis? DR LOVE: Mark, what’s your reaction to the TOPO II data that were
presented as a part of the BCIRG 006 analysis? |
 DR PEGRAM: The TOPO II data from BCIRG 006 are retrospective and
are the result of a subset analysis of an interim analysis of the efficacy data;
ergo, all of the data should be considered exploratory in nature and hypothesis-generating (Press 2005; Slamon 2005). In terms of clinical practice, you have
to understand the caveats and limitations of the data set before you make any
decisions about its applicability to clinical practice.
DR PEGRAM: The TOPO II data from BCIRG 006 are retrospective and
are the result of a subset analysis of an interim analysis of the efficacy data;
ergo, all of the data should be considered exploratory in nature and hypothesis-generating (Press 2005; Slamon 2005). In terms of clinical practice, you have
to understand the caveats and limitations of the data set before you make any
decisions about its applicability to clinical practice.
The topoisomerase story began with Hy Muss’s publication in the mid-1990s of a CALGB trial looking at different doses of doxorubicin-containing
adjuvant chemotherapy (Muss 1994). Among patients with elevated HER2
expression, a dose effect of the different doses of doxorubicin was seen,
whereas in the non-HER2-expressing subset no such effect was evident,
suggesting that patients with HER2-positive disease uniquely benefit from
adjuvant anthracyclines.
This is what really started the whole dogma in clinical practice that patients
with HER2-positive disease uniquely benefit from anthracyclines and why
clinicians began using HER2 as a marker to predict responsiveness to doxorubicin.
However, the question from a research point of view is whether it’s
really HER2 that’s driving this phenotype or something else.
We talked to Giovanni Pauletti at UCLA, who was then mapping the HER2
amplicon, and he, as well as others, pointed out that the TOPO II gene is in
close physical proximity to the HER2 gene on the long arm of chromosome
17 and that, on occasion, it is coamplified with the HER2 gene.
Inasmuch as TOPO II alpha is the target for the anthracyclines, perhaps this
is what’s driving the sensitivity phenotype to doxorubicin rather than HER2
itself.
A number of groups have tested this hypothesis in clinical trials, particularly
in neoadjuvant clinical trials. We knew that we’d have an opportunity to test this hypothesis in the BCIRG trial because we had both an anthracycline- and
a nonanthracycline-containing trastuzumab arm in this three-arm trial, and
amplification of HER2 was an eligibility criterion.
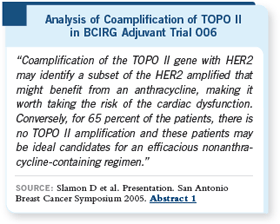 Mike Press has completed the TOPO II analysis for 2,120 of the 3,200 patients
in the BCIRG 006 trial. In this cohort, he found that approximately 35
percent of the patients had coamplification of TOPO II and that this coamplification
seemed to confer a therapeutic advantage to anthracycline-based
trastuzumab regimens.
Mike Press has completed the TOPO II analysis for 2,120 of the 3,200 patients
in the BCIRG 006 trial. In this cohort, he found that approximately 35
percent of the patients had coamplification of TOPO II and that this coamplification
seemed to confer a therapeutic advantage to anthracycline-based
trastuzumab regimens.
Patients with HER2-positive
breast cancer that was not
coamplified for TOPO II,
who constituted two thirds
of the patients, did not appear
to have the same benefit
and therefore may be ideal
candidates for efficacious
nonanthracycline-based
trastuzumab regimens, thus
avoiding potential cardiac
toxicity.
 DR LOVE: Dan, you chaired the San Antonio session where Dr Slamon presented these data, and following
the talk you made the comment that the TOPO II data were not ready for
clinical practice. Could you explain?
DR LOVE: Dan, you chaired the San Antonio session where Dr Slamon presented these data, and following
the talk you made the comment that the TOPO II data were not ready for
clinical practice. Could you explain?
 DR HAYES: There could be a number of reasons for this to be a false-positive
result, and there are a lot of reasons why it could be real. We need longer
follow-up and we need other groups to evaluate this in their own trials.
DR HAYES: There could be a number of reasons for this to be a false-positive
result, and there are a lot of reasons why it could be real. We need longer
follow-up and we need other groups to evaluate this in their own trials.
 DR PEGRAM: With the complete data set from the BCIRG 006 trial of
all 3,200 patients and longer follow-up, the statistical power will be ever
increasing, and it may yield a significant result in the end.
DR PEGRAM: With the complete data set from the BCIRG 006 trial of
all 3,200 patients and longer follow-up, the statistical power will be ever
increasing, and it may yield a significant result in the end.
 Tracks 8-10
Tracks 8-10
 DR LOVE: Dr Burstein, could you summarize the issue of concurrent
versus sequential trastuzumab with chemotherapy? DR LOVE: Dr Burstein, could you summarize the issue of concurrent
versus sequential trastuzumab with chemotherapy? |
 DR BURSTEIN: We have a wealth of data from more than 10,000 patients
assigned to randomized trials of chemotherapy with or without the addition of
trastuzumab, all reported in 2005 and most now in publication (HERA Study
Team 2005; Piccart-Gebhart 2005a, 2005b; Romond 2005a, 2005b). All of
these trials had remarkably consistent results in terms of the improvement in
hazard ratios with the addition of trastuzumab.
DR BURSTEIN: We have a wealth of data from more than 10,000 patients
assigned to randomized trials of chemotherapy with or without the addition of
trastuzumab, all reported in 2005 and most now in publication (HERA Study
Team 2005; Piccart-Gebhart 2005a, 2005b; Romond 2005a, 2005b). All of
these trials had remarkably consistent results in terms of the improvement in
hazard ratios with the addition of trastuzumab.
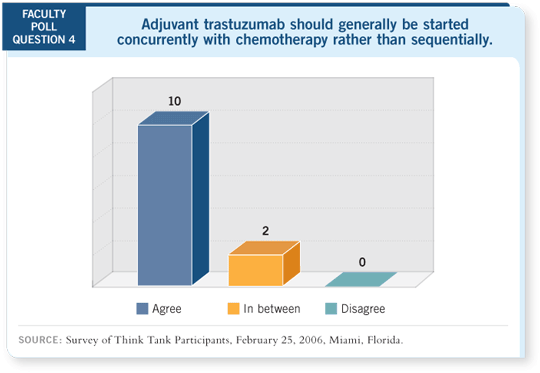
The interesting question has been whether it is better to give adjuvant
trastuzumab concurrently with chemotherapy or sequentially. In the North
American Intergroup trial, NCCTG-N9831, in arm A patients received
chemotherapy alone, in arm B they received AC followed by paclitaxel
followed by trastuzumab, and in arm C the patients received AC followed by
concurrent paclitaxel/trastuzumab and then ongoing trastuzumab.
The analysis comparing arms A and B showed no significant difference in
event-free survival, whereas the preliminary comparison of arms B and C
— concurrent versus sequential therapy — suggested roughly a 40 percent
reduction in the risk of recurrence with concurrent therapy, which was statistically
significant (Perez 2005).
In the HERA trial, the patients were randomly assigned to zero, one or two
years of trastuzumab, and all trastuzumab was given sequentially to chemotherapy.
In contrast to the Intergroup findings, this trial showed roughly a 50
percent reduction in the risk of recurrence with sequential trastuzumab, as
measured by disease-free survival (Piccart-Gebhart 2005).
 DR LOVE: How does one reconcile the N9831 and the HERA data?
DR LOVE: How does one reconcile the N9831 and the HERA data?
 DR BURSTEIN: My own hypotheses are, first, that N9831 remains somewhat
underpowered because of the lack of events. Therefore, it’s possible, if not
probable, that an ongoing analysis of arms A and B — that is, no trastuzumab versus sequential trastuzumab — might have shown more of an advantage.
DR BURSTEIN: My own hypotheses are, first, that N9831 remains somewhat
underpowered because of the lack of events. Therefore, it’s possible, if not
probable, that an ongoing analysis of arms A and B — that is, no trastuzumab versus sequential trastuzumab — might have shown more of an advantage.
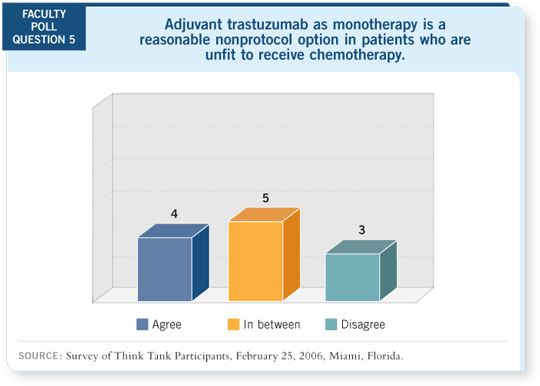
Also, all the patients in the N9831 trial received anthracycline- and taxane-based
therapy, whereas the HERA trial did not specify the adjuvant chemotherapy
to be used.
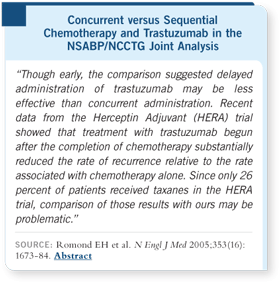 Whereas the vast majority
of patients in HERA
received anthracycline-based
regimens, only one quarter
received a taxane, and those
patients who received an
anthracycline and a taxane
received the least benefit
from trastuzumab.
Whereas the vast majority
of patients in HERA
received anthracycline-based
regimens, only one quarter
received a taxane, and those
patients who received an
anthracycline and a taxane
received the least benefit
from trastuzumab.
My interpretation of the
N9831 and HERA trials
is that concurrent therapy
might be clinically more
efficacious overall than
sequential therapy, and
sequential therapy is only
modestly better than no therapy in patients receiving anthracycline- and taxane-based treatment.
The BCIRG 006 trial also looked at concurrent trastuzumab, and those data
suggest that trastuzumab concurrent with chemotherapy is beneficial (Slamon
2005).
 DR LOVE: Do you feel single-agent trastuzumab is a reasonable option in the
delayed adjuvant setting for patients who did not receive it previously?
DR LOVE: Do you feel single-agent trastuzumab is a reasonable option in the
delayed adjuvant setting for patients who did not receive it previously?
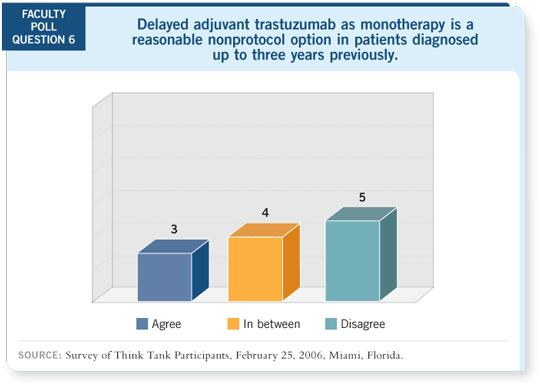
 DR BURSTEIN: For patients who completed anthracycline- and taxane-based
chemotherapy six or 12 months previously and did not receive trastuzumab,
few data suggest that the subsequent addition of trastuzumab will significantly
lengthen their disease-free survival.
DR BURSTEIN: For patients who completed anthracycline- and taxane-based
chemotherapy six or 12 months previously and did not receive trastuzumab,
few data suggest that the subsequent addition of trastuzumab will significantly
lengthen their disease-free survival.
 Track 11
Track 11
 DR LOVE: What about using a chemotherapy/trastuzumab combination
without a taxane? DR LOVE: What about using a chemotherapy/trastuzumab combination
without a taxane? |
 DR WINER: I’m not sure that absolutely every patient with HER2-positive
breast cancer needs to receive AC followed by paclitaxel, particularly patients
who don’t want to receive a taxane or those with a lower risk of recurrence.
Based on the HERA data, I believe it’s reasonable to give four cycles of AC followed by a year of trastuzumab. The risk reduction is every bit as large as in
the US trials.
DR WINER: I’m not sure that absolutely every patient with HER2-positive
breast cancer needs to receive AC followed by paclitaxel, particularly patients
who don’t want to receive a taxane or those with a lower risk of recurrence.
Based on the HERA data, I believe it’s reasonable to give four cycles of AC followed by a year of trastuzumab. The risk reduction is every bit as large as in
the US trials.
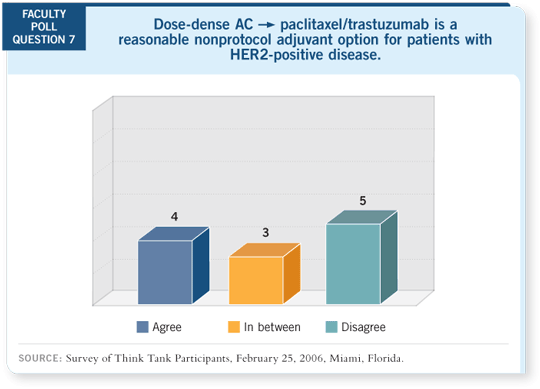
 DR LOVE: Can anyone identify a patient for whom you would consider
adjuvant trastuzumab without chemotherapy?
DR LOVE: Can anyone identify a patient for whom you would consider
adjuvant trastuzumab without chemotherapy?
 DR VOGEL: I just had a patient with high-risk, node-negative breast cancer
and a concomitant Epstein-Barr virus infection and hepatitis C. She is very
well informed and deathly afraid of immunosuppression secondary to chemotherapy,
and no one could convince her to receive chemotherapy.
DR VOGEL: I just had a patient with high-risk, node-negative breast cancer
and a concomitant Epstein-Barr virus infection and hepatitis C. She is very
well informed and deathly afraid of immunosuppression secondary to chemotherapy,
and no one could convince her to receive chemotherapy.
For this patient, even in the absence of data, I thought it was better to administer
trastuzumab monotherapy than not to give her trastuzumab.
 DR LOVE: What about patients for whom you previously wouldn’t have
administered adjuvant chemotherapy because of age or comorbidities?
DR LOVE: What about patients for whom you previously wouldn’t have
administered adjuvant chemotherapy because of age or comorbidities?
 DR VOGEL: I would consider trastuzumab alone.
DR VOGEL: I would consider trastuzumab alone.
 DR OSBORNE: I have not given single-agent trastuzumab in the adjuvant
setting in practice, but I’m a believer in targeted therapy.
DR OSBORNE: I have not given single-agent trastuzumab in the adjuvant
setting in practice, but I’m a believer in targeted therapy.
I believe tumors are driven by certain pathways and that, if you block that
pathway, you will kill the tumor. We’ve seen that now with hormonal therapy.
For patients with ER/PR-positive tumors, except for those tumors that are resistant, endocrine therapy is very good and chemotherapy doesn’t add
anything or it adds, at most, only a tiny benefit. I believe we will find that in
the future, for patients with HER2-positive disease, HER2-targeted therapy
without chemotherapy will be all we need.
Select publications

