
| Tracks 1-21 |
| Track 1 |
Optimal long-term treatment
strategy for postmenopausal
patients with ER-positive disease |
| Track 2 |
Side effects associated with
aromatase inhibitors versus
tamoxifen |
| Track 3 |
Rationale for use of an up-front
aromatase inhibitor versus
switching at two to three years |
| Track 4 |
Meta-analysis of adjuvant
aromatase inhibitor trials |
| Track 5 |
Lack of long-term toxicity data
with aromatase inhibitors |
| Track 6 |
Rationale for incomparability of
up-front versus switching data
in trials of adjuvant aromatase
inhibitors |
| Track 7 |
Use of computer models to
select optimal adjuvant hormonal
therapy |
| Track 8 |
Potential benefit of sequencing
an aromatase inhibitor after two
to three years of tamoxifen |
| Track 9 |
Safety of long-term administration
of an aromatase inhibitor |
| Track 10 |
Duration of adjuvant hormonal
therapy |
|
| Track 11 |
Sequencing tamoxifen after
adjuvant aromatase inhibitors |
| Track 12 |
Role of HER2 and PR as
predictive factors for tamoxifen |
| Track 13 |
Effect of HER2 and PR status on
response to aromatase inhibitors |
| Track 14 |
Clinical use of HER2 and PR to
select adjuvant hormonal therapy |
| Track 15 |
Selection of optimal adjuvant
hormonal therapy |
| Track 16 |
Adjuvant hormonal therapy for
patients with low-risk disease |
| Track 17 |
Differential effects of hormonal
therapies based on ER and
PR status |
| Track 18 |
Selection of hormonal therapy for
premenopausal women with ER-positive
disease |
| Track 19 |
Aromatase inhibitors in
combination with ovarian
suppression for premenopausal
patients |
| Track 20 |
Switching from tamoxifen to an
aromatase inhibitor for premenopausal
patients who become
amenorrheic |
| Track 21 |
Clinical use of ovarian
suppression and an aromatase
inhibitor |
|
|
Select Excerpts from the Discussion
 Tracks 1, 3
Tracks 1, 3
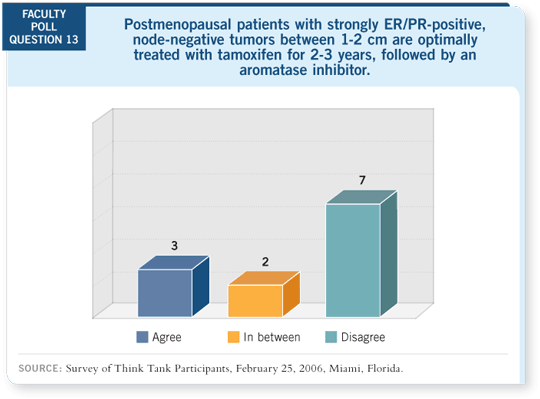
 DR LOVE: Aman, what do you consider the optimal adjuvant endocrine
approach for postmenopausal patients with ER/PR-positive disease? DR LOVE: Aman, what do you consider the optimal adjuvant endocrine
approach for postmenopausal patients with ER/PR-positive disease? |
 DR BUZDAR: I believe the most effective therapy for patients with newly diagnosed breast cancer should be offered up front. We are now looking at
more than 30,000 patients who have been randomly assigned in the aromatase
inhibitor trials, which clearly demonstrate that it doesn’t matter where we
put the aromatase inhibitors;
they have better efficacy, a
reduced risk of recurrence
and a better safety profile.
The most effective therapy
should be offered up front to
these patients.
DR BUZDAR: I believe the most effective therapy for patients with newly diagnosed breast cancer should be offered up front. We are now looking at
more than 30,000 patients who have been randomly assigned in the aromatase
inhibitor trials, which clearly demonstrate that it doesn’t matter where we
put the aromatase inhibitors;
they have better efficacy, a
reduced risk of recurrence
and a better safety profile.
The most effective therapy
should be offered up front to
these patients.
If we look at the ATAC
trial, which has the longest
follow-up — and all of the
other studies show the same
thing — among patients with
receptor-positive disease,
initial endocrine therapy
with an aromatase inhibitor
reduces the risk of recurrence
by 26 percent. The absolute
number, at six years, is about
3.7 percent more women alive and free of disease if we start with an aromatase inhibitor (Howell 2005).
BIG 1-98 shows a similar type of benefit (Thürlimann 2005).
 At times, physicians become confused when they see the proportional reductions
in the studies that were initiated after two to three years or after the
completion of five years of adjuvant tamoxifen therapy. You are comparing
apples to oranges. You cannot take that type of data and compare it to the up-front
data because in those types of studies, the patient population is different
because the patients at high risk have relapsed.
At times, physicians become confused when they see the proportional reductions
in the studies that were initiated after two to three years or after the
completion of five years of adjuvant tamoxifen therapy. You are comparing
apples to oranges. You cannot take that type of data and compare it to the up-front
data because in those types of studies, the patient population is different
because the patients at high risk have relapsed.
We can also reduce the risk of recurrence if we start an aromatase inhibitor
after two to three years of tamoxifen. This is a proportional reduction because
the continuation of tamoxifen therapy is inferior to switching the patient to an
aromatase inhibitor (Boccardo 2005; Coombes 2004; Jakesz 2005). The ATAC
data show about 40 additional events in the first 2.5 years, which include
distant and local recurrences (Baum 2003). I am not aware of any way to select
those patients to whom we can safely offer tamoxifen therapy.
 DR RAVDIN: Disease-free survival is always better on the aromatase inhibitor
arm in all these trials, and the number of deaths is small and not statistically
significant. When you have a disease-free survival advantage, that means
overall you have more patients surviving. Irrespective of whether some of
those patients drop off because of toxicity, patients on average are doing better
at later time points.
DR RAVDIN: Disease-free survival is always better on the aromatase inhibitor
arm in all these trials, and the number of deaths is small and not statistically
significant. When you have a disease-free survival advantage, that means
overall you have more patients surviving. Irrespective of whether some of
those patients drop off because of toxicity, patients on average are doing better
at later time points.
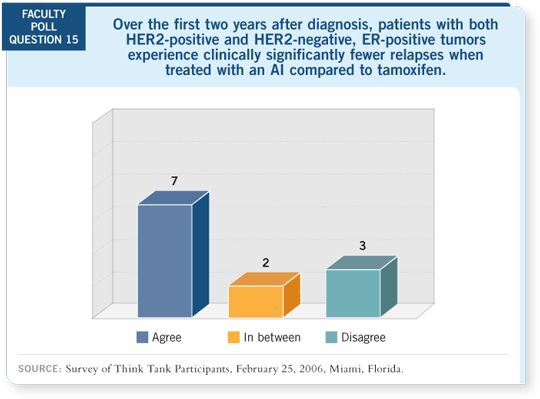
 Tracks 4, 5, 7
Tracks 4, 5, 7
 DR LOVE: What about survival in trials of aromatase inhibitors compared
to tamoxifen? DR LOVE: What about survival in trials of aromatase inhibitors compared
to tamoxifen? |
 DR BUZDAR: Richard Gray took the published events in the adjuvant
aromatase inhibitor trials — he did not have access to individual data points
or the patients’ information — and assessed disease-free survival and recurrences.
Then he assessed deaths from cancer and noncompeting causes. Twenty
percent fewer breast cancer deaths occurred among the patients treated with
the aromatase inhibitors compared to the patients who were on tamoxifen or
placebo, and the confidence interval did not include one.
DR BUZDAR: Richard Gray took the published events in the adjuvant
aromatase inhibitor trials — he did not have access to individual data points
or the patients’ information — and assessed disease-free survival and recurrences.
Then he assessed deaths from cancer and noncompeting causes. Twenty
percent fewer breast cancer deaths occurred among the patients treated with
the aromatase inhibitors compared to the patients who were on tamoxifen or
placebo, and the confidence interval did not include one.
Also, without question, every study shows a disease-free survival advantage
with the aromatase inhibitors compared to tamoxifen, and the side effects are
predictable compared to the unpredictable side effects that cannot be prevented
with tamoxifen.
 DR OSBORNE: I don’t think we can be so dogmatic about this issue. We have
25 million patient-years of exposure to tamoxifen. I don’t know how many we
have for an aromatase inhibitor, but it’s probably a twentieth. We don’t know
what’s going to happen after five years with an aromatase inhibitor. You can guess that there won’t be any more long-term side effects, but we don’t know.
DR OSBORNE: I don’t think we can be so dogmatic about this issue. We have
25 million patient-years of exposure to tamoxifen. I don’t know how many we
have for an aromatase inhibitor, but it’s probably a twentieth. We don’t know
what’s going to happen after five years with an aromatase inhibitor. You can guess that there won’t be any more long-term side effects, but we don’t know.
We can’t make dogmatic statements about which sequence is best in the
absence of any information on toxicity or benefit. Given the information from
the ATAC trial with hormone receptor analyses and the models suggesting the
possibility of a huge benefit for tamoxifen followed by an aromatase inhibitor,
depending on what happens after five years, I think we have to be open to the
idea that either of these strategies might, in the end, be worthwhile.
 DR BURSTEIN: I continue to find MA17 to be the most intellectually fascinating
of the adjuvant endocrine trials because it has shown us two things.
First, it has shown that treatment beyond five years changes the natural history
of the disease (Goss 2005a). That’s been a very powerful finding. Second, the
more recent data suggest that even gaps in the treatment can be followed up by
late interventions (Goss 2005b). This is forcing us to realize that we’re talking
about a disease in which the outcomes matter over years five, 10 and 15,
something that the most recent overview also suggested.
DR BURSTEIN: I continue to find MA17 to be the most intellectually fascinating
of the adjuvant endocrine trials because it has shown us two things.
First, it has shown that treatment beyond five years changes the natural history
of the disease (Goss 2005a). That’s been a very powerful finding. Second, the
more recent data suggest that even gaps in the treatment can be followed up by
late interventions (Goss 2005b). This is forcing us to realize that we’re talking
about a disease in which the outcomes matter over years five, 10 and 15,
something that the most recent overview also suggested.
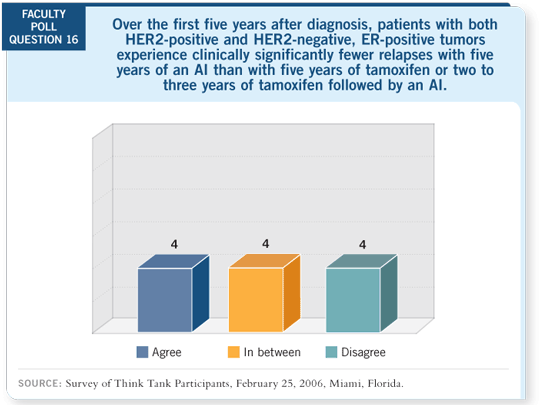
 Tracks 10-15
Tracks 10-15
 DR LOVE: With regard to the duration of adjuvant therapy with an
aromatase inhibitor, I assume people who start aromatase inhibitors up
front are stopping after five years. Cliff, could you comment on this issue? DR LOVE: With regard to the duration of adjuvant therapy with an
aromatase inhibitor, I assume people who start aromatase inhibitors up
front are stopping after five years. Cliff, could you comment on this issue? |
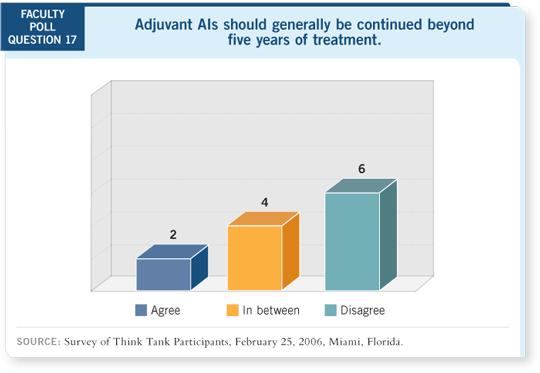
 DR HUDIS: The interesting
thing about MA17 (Goss
2005a) and now MA17R
(Goss 2005b) is the notion
that you can reduce that
hazard rate at almost any
time in those first 10 years
and maybe longer. This
is motivation for chronic
suppressive therapy. I have a
bias toward leaving patients
on a therapy that they’re
tolerating.
DR HUDIS: The interesting
thing about MA17 (Goss
2005a) and now MA17R
(Goss 2005b) is the notion
that you can reduce that
hazard rate at almost any
time in those first 10 years
and maybe longer. This
is motivation for chronic
suppressive therapy. I have a
bias toward leaving patients
on a therapy that they’re
tolerating.
 We stop tamoxifen for two
reasons. One, we had clear
evidence of accumulating
toxicity, which we have yet
to garner with the aromatase inhibitors, but it could be there. Two, we had
one randomized trial that failed to demonstrate benefit (Fisher 2001).
We stop tamoxifen for two
reasons. One, we had clear
evidence of accumulating
toxicity, which we have yet
to garner with the aromatase inhibitors, but it could be there. Two, we had
one randomized trial that failed to demonstrate benefit (Fisher 2001).
 DR RAVDIN: I believe it’s analogous to the situation with five years of
tamoxifen. Patients were reluctant to stop tamoxifen when we didn’t have any data, and many elected to stay on the therapy. In this situation, we do have
randomized trials that are addressing this question. I trust that the Data and
Safety Monitoring Committees will stop those trials, the way they stopped the
tamoxifen trials, if evidence appears of bad effects.
DR RAVDIN: I believe it’s analogous to the situation with five years of
tamoxifen. Patients were reluctant to stop tamoxifen when we didn’t have any data, and many elected to stay on the therapy. In this situation, we do have
randomized trials that are addressing this question. I trust that the Data and
Safety Monitoring Committees will stop those trials, the way they stopped the
tamoxifen trials, if evidence appears of bad effects.
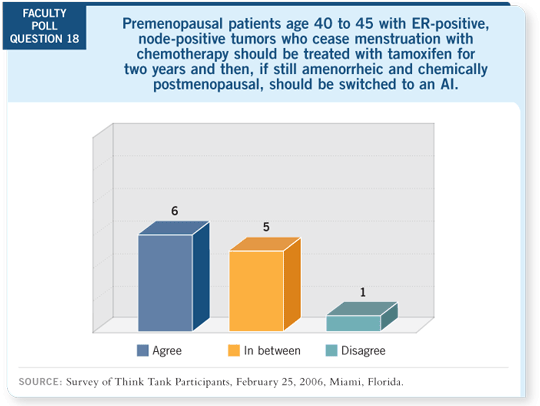
 DR BURSTEIN: We’ve created a very awkward situation. If a woman begins
an aromatase inhibitor up front, she receives five years of adjuvant endocrine
therapy. If she were to come to you having started on tamoxifen, then after
five years of treatment she would receive 10 years of adjuvant endocrine
therapy. If she switched somewhere in between, she would receive either
five or 10 years, depending on how you look at the literature. That seems
somehow inconsistent.
DR BURSTEIN: We’ve created a very awkward situation. If a woman begins
an aromatase inhibitor up front, she receives five years of adjuvant endocrine
therapy. If she were to come to you having started on tamoxifen, then after
five years of treatment she would receive 10 years of adjuvant endocrine
therapy. If she switched somewhere in between, she would receive either
five or 10 years, depending on how you look at the literature. That seems
somehow inconsistent.
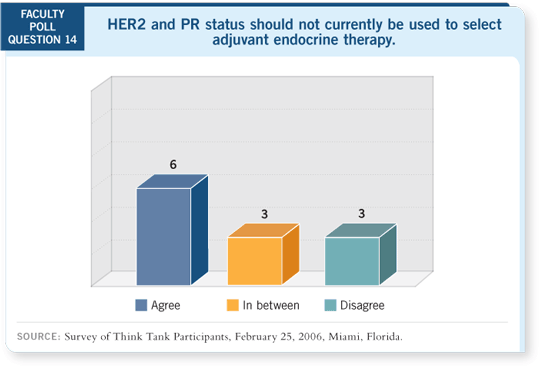
 Tracks 12-14
Tracks 12-14
 DR LOVE: Kent, can you summarize what we know about predictors of
response to hormonal therapy? DR LOVE: Kent, can you summarize what we know about predictors of
response to hormonal therapy? |
 DR OSBORNE: One important issue is whether HER2 overexpression and
PR loss predict for less benefit from tamoxifen than from an aromatase inhibitor.
To me, the data are overwhelming that PR status predicts for response to
tamoxifen.
DR OSBORNE: One important issue is whether HER2 overexpression and
PR loss predict for less benefit from tamoxifen than from an aromatase inhibitor.
To me, the data are overwhelming that PR status predicts for response to
tamoxifen.
In a prospectively designed SWOG trial published by Peter, patients with
metastatic disease were treated with tamoxifen. The trial was designed to
address the value of PR status. On multivariate analysis, PR status was found
to be an independent predictor (Ravdin 1992).
That was the first prospective trial following another five or 10 studies
published in the early 1980s and late 1970s suggesting that patients with PR-negative
disease responded less well to tamoxifen.
What about HER2 overexpression and tamoxifen? Most, but not all, studies
show less benefit if HER2 is overexpressed. Preclinical studies strongly
support the clinical data. So I tend to believe the majority of the clinical data,
along with the biology, that HER2 does predict for less responsiveness to
tamoxifen.
We have very little data with the aromatase inhibitors. We have three separate
neoadjuvant trials (Ellis 2001; Smith 2005; Zhu 2004) and a fourth (Dixon
2004) from Mike Dixon’s group in Edinburgh that show very similar results.
Whether it is letrozole or anastrozole, the responses are really quite good for
patients with HER2-positive disease.
 DR SLEDGE: I find the ER-PR data interesting biologically. Having said
that, I don’t know how much real-world relevance it has because I can’t pick
out any population of patients in whom tamoxifen does better than an aromatase inhibitor. Because of that, my default — unless it’s going to be the oddball
patient who can’t tolerate an aromatase inhibitor for some reason — will be to
use an aromatase inhibitor.
DR SLEDGE: I find the ER-PR data interesting biologically. Having said
that, I don’t know how much real-world relevance it has because I can’t pick
out any population of patients in whom tamoxifen does better than an aromatase inhibitor. Because of that, my default — unless it’s going to be the oddball
patient who can’t tolerate an aromatase inhibitor for some reason — will be to
use an aromatase inhibitor.
 Tracks 19, 20
Tracks 19, 20
 DR LOVE: Eric, can you comment on our current investigational strategies
for premenopausal patients with ER-positive disease? DR LOVE: Eric, can you comment on our current investigational strategies
for premenopausal patients with ER-positive disease? |
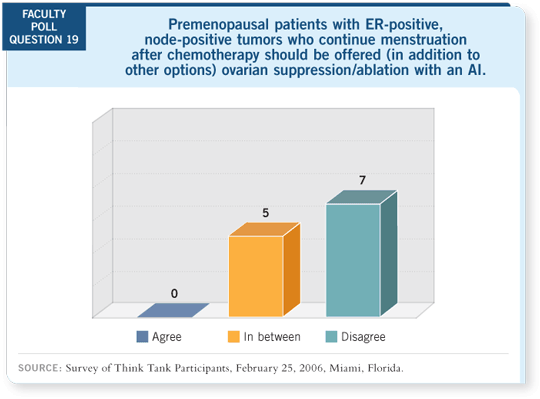
 DR WINER: The issue of ovarian suppression with an aromatase inhibitor is
being addressed in the SOFT and TEXT trials. At least some reason exists to
be concerned that this could possibly be an inferior strategy.
DR WINER: The issue of ovarian suppression with an aromatase inhibitor is
being addressed in the SOFT and TEXT trials. At least some reason exists to
be concerned that this could possibly be an inferior strategy.
In a woman who has a high level of estrogen in the premenopausal state, the
estrogen levels go down after she receives ovarian suppression. Then adding
an aromatase inhibitor and taking a woman down to extremely low levels of
estrogen may add benefit. It’s also possible that taking those two steps down is,
in fact, no better than a single step.
Of course, from a toxicity standpoint — as I think we’re learning from
both TEXT and SOFT — that deep plunge into not only menopause but
menopause and an aromatase inhibitor is a pretty tough maneuver for most of
these patients. So for premenopausal women, I would strongly argue against
using ovarian suppression and an aromatase inhibitor as an up-front strategy
outside of a clinical trial.
What about the use of an aromatase inhibitor for a woman who is premenopausal
at diagnosis, stops cycling soon after diagnosis and is now on tamoxifen
for two years? This situation is much more analogous to the postmenopausal
woman. She has now been without premenopausal levels of estrogen for two
years.
It is more likely that substituting an aromatase inhibitor for tamoxifen after
two years could be of additional benefit. We don’t know that from any of the
clinical trials that have been performed, but it seems more rational.
However, we’ve all seen in practice — and Hal actually has a whole series of
these women — patients who have been without menstrual cycles for a couple
of years go off tamoxifen and start cycling again.
 DR HUDIS: I believe we’re wrong to treat patients with aromatase inhibitors
who are in their mid forties and had no periods while on tamoxifen. The
random sampling of their estradiol and FSH does nothing to change that.
DR HUDIS: I believe we’re wrong to treat patients with aromatase inhibitors
who are in their mid forties and had no periods while on tamoxifen. The
random sampling of their estradiol and FSH does nothing to change that.
 DR OSBORNE: We’ve started measuring them, and I’m totally flabbergasted
by the number of patients who are amenorrheic, even in their late forties, early
fifties, who still have premenopausal levels of estrogen.
DR OSBORNE: We’ve started measuring them, and I’m totally flabbergasted
by the number of patients who are amenorrheic, even in their late forties, early
fifties, who still have premenopausal levels of estrogen.
 DR BURSTEIN: The point is that amenorrhea is menopause, but that’s not a very good definition for treating patients with aromatase inhibitors. We began
to notice some patients — all of whom were women in their forties who had
chemotherapy-induced amenorrhea — who were thought biochemically or on
strong clinical grounds to be truly menopausal and were put on an aromatase
inhibitor.
DR BURSTEIN: The point is that amenorrhea is menopause, but that’s not a very good definition for treating patients with aromatase inhibitors. We began
to notice some patients — all of whom were women in their forties who had
chemotherapy-induced amenorrhea — who were thought biochemically or on
strong clinical grounds to be truly menopausal and were put on an aromatase
inhibitor.
Usually, within six to 18 months they began to have menstruation again or
had biochemical evidence of residual ovarian function, suggesting that they
were not obtaining a therapeutic gain from an aromatase inhibitor.
Select publications

