
| Tracks 1-12 |
| Track 1 |
Prophylactic growth factor
support to prevent febrile
neutropenia with adjuvant
chemotherapy |
| Track 2 |
Clinical use of prophylactic
growth factor support in the
adjuvant setting |
| Track 3 |
Defining an acceptable level
of risk for the development of
neutropenia |
| Track 4 |
Use of docetaxel/cyclophosphamide
versus AC in the
adjuvant setting |
| Track 5 |
Clinical trial data examining the
benefit of adjuvant chemotherapy
for patients with ER-positive
disease |
| Track 6 |
Adjuvant TAC versus dose-dense
AC/paclitaxel for patients with ER-positive
disease |
|
| Track 7 |
Quality control in hormone
receptor testing |
| Track 8 |
BCIRG 005: Adjuvant TAC versus
AC followed by docetaxel |
| Track 9 |
Amenorrhea with TAC versus
AC/paclitaxel |
| Track 10 |
Benefit of adjuvant chemotherapy
for patients with ER-positive
disease |
| Track 11 |
ECOG-PACCT-1: Adjuvant
hormonal therapy with or without
chemotherapy based on the
Oncotype DX recurrence score |
| Track 12 |
Benefit of chemotherapy in
addition to hormonal therapy |
|
|
Select Excerpts from the Discussion
 Tracks 1, 2
Tracks 1, 2
 DR LOVE: Chuck, can you summarize your data with regard to the use
of growth factor support during the administration of adjuvant chemotherapy? DR LOVE: Chuck, can you summarize your data with regard to the use
of growth factor support during the administration of adjuvant chemotherapy? |
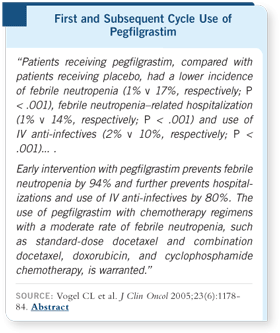
 DR VOGEL: In our study, docetaxel was chosen as a representative regimen
that could cause somewhere around a 20 percent risk of febrile neutropenia at
100 mg/m2. All three endpoints — febrile neutropenia, febrile neutropenia-related
hospitalization and use of anti-infectives — showed dramatic improvement
with the addition of pegfilgrastim (Vogel 2005).
DR VOGEL: In our study, docetaxel was chosen as a representative regimen
that could cause somewhere around a 20 percent risk of febrile neutropenia at
100 mg/m2. All three endpoints — febrile neutropenia, febrile neutropenia-related
hospitalization and use of anti-infectives — showed dramatic improvement
with the addition of pegfilgrastim (Vogel 2005).
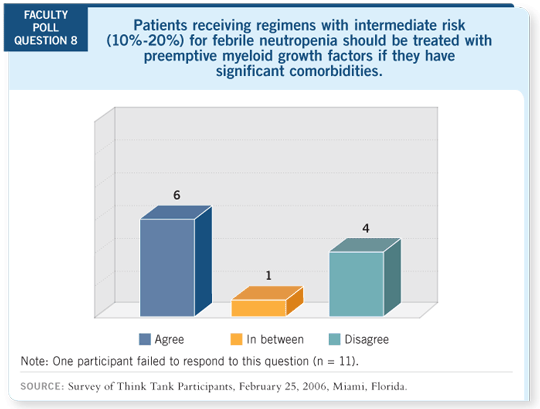
Most people would agree with the new NCCN guidelines (Lyman 2005)
stating that prophylactic growth factors should be used for patients with
greater than 20 percent risk of febrile neutropenia. The use of prophylactic
growth factors should also be considered in the intermediate-risk group, 10 to 20 percent. Patients at
low risk should not receive
growth factors.
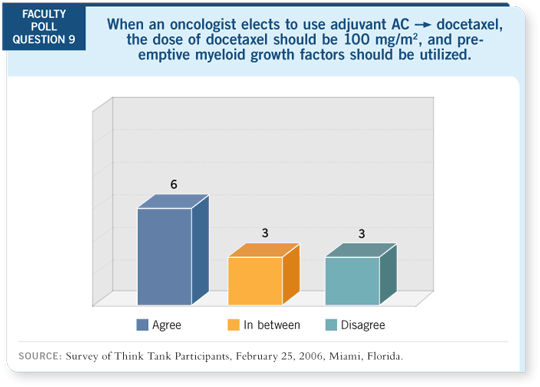
AC followed by docetaxel,
AT and TAC (Martin 2005)
all have very high febrile
neutropenia rates, and
prophylactic growth factors
should be strongly considered
with these regimens. AC is
considered an intermediate-risk
regimen, as is docetaxel/capecitabine.
 FAC, FEC and TC are
regimens associated with
borderline to low febrile
neutropenia rates. Certainly,
dose densification of any
of these would be a reason
to use prophylactic pegfilgrastim,
as would the avoidance of dose reductions and delays.
FAC, FEC and TC are
regimens associated with
borderline to low febrile
neutropenia rates. Certainly,
dose densification of any
of these would be a reason
to use prophylactic pegfilgrastim,
as would the avoidance of dose reductions and delays.
A third reason to consider it would be the risk factors that may cause patients
to be at risk for febrile neutropenia.
 DR BURSTEIN: I believe most of us would have a hard time consenting to a
regimen associated with a one in five chance of a patient being hospitalized
with febrile neutropenia compared to one that wasn’t, simply for the administration
of prophylaxis. So I don’t find a problem with the recommendation for
prophylactic treatment at 15 to 20 percent risk.
DR BURSTEIN: I believe most of us would have a hard time consenting to a
regimen associated with a one in five chance of a patient being hospitalized
with febrile neutropenia compared to one that wasn’t, simply for the administration
of prophylaxis. So I don’t find a problem with the recommendation for
prophylactic treatment at 15 to 20 percent risk.
The problem is that we as a community haven’t defined an acceptable level of
febrile neutropenia. For instance, with nausea and vomiting, we all agree the
desired goal is zero, so we liberally use prophylaxis.
For cancer pain, the goal is zero, so we liberally use pain medicine. We haven’t
said what we’re willing to tolerate in the way of febrile neutropenia risk.
The only other anecdote I can offer is that as I administer AC every three
weeks for patients destined to receive adjuvant trastuzumab, I’m struck by how
many patients end up having dose delays and tweaks.
It’s clearly more toxic than using dose-dense AC followed by paclitaxel with
growth factor support.
This hasn’t caused me to use G-CSF prophylactically in these settings, but it is impressive how predictable and clockwork-like every two-week AC with
growth factor support is compared to other treatments.
I believe if you asked patients whether they would take a growth factor for a
four percent decrease in their chance of febrile neutropenia, they’d all say yes.
Whether that is cost-effective is a totally different question.
 Track 4
Track 4
 DR LOVE: Cliff, what’s your take on the adjuvant trial data that Steve
Jones presented at San Antonio comparing docetaxel/cyclophosphamide
to AC? DR LOVE: Cliff, what’s your take on the adjuvant trial data that Steve
Jones presented at San Antonio comparing docetaxel/cyclophosphamide
to AC? |
 DR HUDIS: We vary in our acceptance of new regimens based on the
clinical endpoint bar they cross. Sometimes disease-free survival is absolutely
fine. In other settings, people go ballistic if you don’t have overall survival
data as well. Here is a setting in which, at the second analysis, we have an
improvement in disease-free survival and we still don’t have an overall survival
advantage.
DR HUDIS: We vary in our acceptance of new regimens based on the
clinical endpoint bar they cross. Sometimes disease-free survival is absolutely
fine. In other settings, people go ballistic if you don’t have overall survival
data as well. Here is a setting in which, at the second analysis, we have an
improvement in disease-free survival and we still don’t have an overall survival
advantage.
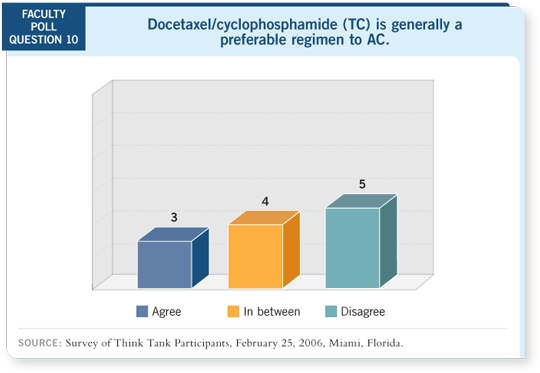
I recall that when these data were presented for the first time at ASCO a
couple of years ago, with widely separated curves, we were told that the trial
would never be statistically significant because it was underpowered. So this result came as a bit of a surprise at San Antonio.
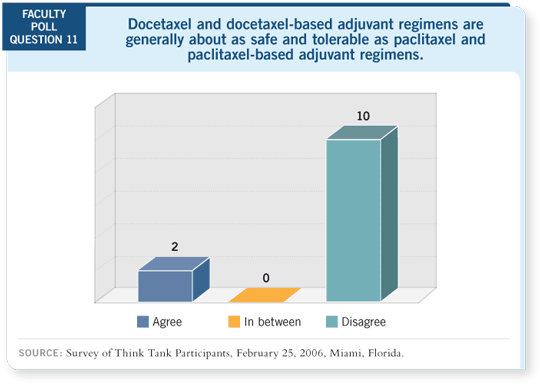
Having said all that, I have a bias. If I were a user of AC, I’d have a hard time
not justifying TC. If nothing else, it’s no more acutely toxic, by the randomized
comparison, and it certainly should eliminate the small but meaningful
long-term risk of cardiac toxicity. It will be interesting to see the long-term
leukemia risk without the anthracycline.
 DR RAVDIN: The hazard ratio for recurrence shows a 24 percent proportional
advantage in survival for docetaxel/cyclophosphamide, which is as big a step as
we usually take in our clinical trials, and it shows a 36 percent improvement
in disease-free survival. I believe the improvement in overall survival is real,
and the correct interpretation isn’t that it doesn’t show a survival advantage but
that it’s underpowered to show a 24 percent advantage.
DR RAVDIN: The hazard ratio for recurrence shows a 24 percent proportional
advantage in survival for docetaxel/cyclophosphamide, which is as big a step as
we usually take in our clinical trials, and it shows a 36 percent improvement
in disease-free survival. I believe the improvement in overall survival is real,
and the correct interpretation isn’t that it doesn’t show a survival advantage but
that it’s underpowered to show a 24 percent advantage.
 DR WINER: It’s one study, not multiple studies, and it comes on the heels of
the negative ECOG trial of AC versus AT (Goldstein 2005). I have a difficult
time reconciling those two trials. If, in fact, the substitution of docetaxel
for cyclophosphamide wasn’t better, I find it certainly not inconceivable but a
little funny that it’s better than an anthracycline.
DR WINER: It’s one study, not multiple studies, and it comes on the heels of
the negative ECOG trial of AC versus AT (Goldstein 2005). I have a difficult
time reconciling those two trials. If, in fact, the substitution of docetaxel
for cyclophosphamide wasn’t better, I find it certainly not inconceivable but a
little funny that it’s better than an anthracycline.
TC is a fine regimen to use, but I don’t believe that it has to be the standard
regimen to replace AC at the moment. I haven’t chosen to use it as a standard
regimen other than for patients for whom I don’t want to administer an anthracycline.
 DR HUDIS: This makes a point that there’s no evidence that TC is inferior
to AC, and it may well be safer in the long term. So I would feel little risk in
substituting docetaxel for doxorubicin. I never use AC alone, so it’s easy for
me to say that. In my hands, everybody who receives AC also receives paclitaxel.
DR HUDIS: This makes a point that there’s no evidence that TC is inferior
to AC, and it may well be safer in the long term. So I would feel little risk in
substituting docetaxel for doxorubicin. I never use AC alone, so it’s easy for
me to say that. In my hands, everybody who receives AC also receives paclitaxel.
 DR HAYES: If someone called me and said, “I’m going to use TC instead of
AC,” I would say, “I believe that’s a perfectly fine regimen.”
DR HAYES: If someone called me and said, “I’m going to use TC instead of
AC,” I would say, “I believe that’s a perfectly fine regimen.”
 Tracks 5, 6
Tracks 5, 6
 DR LOVE: Cliff, ER status and response to chemotherapy have become a
controversial issue. What’s your viewpoint? DR LOVE: Cliff, ER status and response to chemotherapy have become a
controversial issue. What’s your viewpoint? |
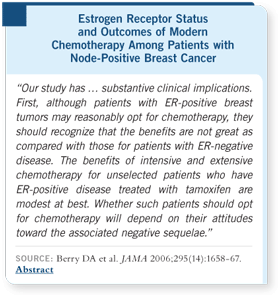
 DR HUDIS: Don Berry started the discussion of the impact of ER status on
chemotherapy outcomes in the modern era by performing an unplanned retrospective
analysis of CALGB trials on the basis of ER status (Berry 2006).
DR HUDIS: Don Berry started the discussion of the impact of ER status on
chemotherapy outcomes in the modern era by performing an unplanned retrospective
analysis of CALGB trials on the basis of ER status (Berry 2006).
 He initially presented his three-study analysis at San Antonio in 2004, and
compared the high-dose every four-week CAF regimen to the standard AC
arm of CALGB-9344. He then studied the AC
He initially presented his three-study analysis at San Antonio in 2004, and
compared the high-dose every four-week CAF regimen to the standard AC
arm of CALGB-9344. He then studied the AC  paclitaxel arm of 9344 against the standard arm of
the dose-dense 9741 trial.
paclitaxel arm of 9344 against the standard arm of
the dose-dense 9741 trial.
For patients with ER-negative
disease, the hazard
for disease-free survival was
significantly improved with
each one of these steps —
better CAF, addition of paclitaxel,
dose-dense scheduling.
Adding up the overall impact
for ER-negative breast
cancer, we see a profound
chemotherapy effect.
In the subset of patients
with ER-positive disease,
the difference in each one of
these steps was not statistically
significant, but they
were always favorable.
The point estimate for
benefit is half the size for the
patients with ER-positive disease compared to those with ER-negative disease.
It is likely that it is still favorable, although the confidence interval does not
exclude the possibility of no benefit at all.
To some degree, this has been wildly overinterpreted as suggesting that
chemotherapy doesn’t work in patients with ER-positive disease.
It simply doesn’t say that. It says that the magnitude of the benefit is likely to
be much smaller than for those with ER-poor disease.
The important point is that when people say that the addition of dose-dense
scheduling in 9741 doesn’t yield much among patients with ER-positive
disease, they’re really not comparing apples to apples when they then look at
the TAC-FAC data (Martin 2005).
 The TAC-FAC trial demonstrated hazard rates for risk reductions, which
looked about the same in the ER-positives and the ER-negatives. The FAC
control arm, of course, includes no paclitaxel or docetaxel.
The TAC-FAC trial demonstrated hazard rates for risk reductions, which
looked about the same in the ER-positives and the ER-negatives. The FAC
control arm, of course, includes no paclitaxel or docetaxel.
You can’t say that each individual step is or is not significant vis-à-vis another
separate randomized trial. You can’t compare these regimens head to head.
If you were to argue that you know to utilize TAC instead of dose-dense
AC paclitaxel in a patient with ER-positive, node-positive disease, then
you’re presuming to know the results of NSABP-B-38.
paclitaxel in a patient with ER-positive, node-positive disease, then
you’re presuming to know the results of NSABP-B-38.
I would argue that there is equipoise on this question and that either regimen is entirely appropriate for
patients with ER-positive
disease.
 Tracks 9, 11, 12
Tracks 9, 11, 12
 DR LOVE: Eric, what are
your thoughts about the
influence of ER status
on the effects of chemotherapy,
particularly
with the newer adjuvant
regimens? DR LOVE: Eric, what are
your thoughts about the
influence of ER status
on the effects of chemotherapy,
particularly
with the newer adjuvant
regimens? |
 DR WINER: It’s very hard
for me to get more excited
about TAC as opposed to
dose-dense AC followed
by paclitaxel. I believe the
bottom line is that if you take all patients with ER-positive breast cancer,
the benefits of chemotherapy are dramatically less than in patients with ER-negative
disease.
DR WINER: It’s very hard
for me to get more excited
about TAC as opposed to
dose-dense AC followed
by paclitaxel. I believe the
bottom line is that if you take all patients with ER-positive breast cancer,
the benefits of chemotherapy are dramatically less than in patients with ER-negative
disease.
Almost certainly, some groups of women with ER-positive breast cancer
derive no benefit and others probably derive every bit as much benefit as the
ER-negative group. It’s not going to be chemotherapy-agent-specific, particularly
when we get down to the level of taxanes.
 DR OSBORNE: This is such an important question because 60 percent of
all patients have ER-positive/PR-positive disease. Will anyone conduct a
randomized trial of chemotherapy versus no chemotherapy or endocrine
therapy alone versus the addition of chemotherapy in that subgroup?
DR OSBORNE: This is such an important question because 60 percent of
all patients have ER-positive/PR-positive disease. Will anyone conduct a
randomized trial of chemotherapy versus no chemotherapy or endocrine
therapy alone versus the addition of chemotherapy in that subgroup?
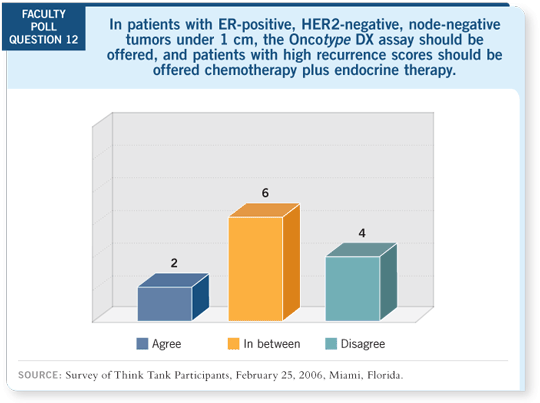
 DR HAYES: The patients with node-negative, ER-positive disease in the
TAILORx, or ECOG-PACCT-1, study will all be profiled by the Oncotype
DXTM assay. Those patients with a good recurrence score of 11 or lower will
receive hormone therapy only.
DR HAYES: The patients with node-negative, ER-positive disease in the
TAILORx, or ECOG-PACCT-1, study will all be profiled by the Oncotype
DXTM assay. Those patients with a good recurrence score of 11 or lower will
receive hormone therapy only.
Those with a high recurrence score of 25 and higher will all receive hormone
therapy and chemotherapy of “dealer’s choice.” Those in the intermediate
group will be randomly assigned to receive chemotherapy or not (investigator’s
choice). They then will all receive hormone therapy, also at the investigator’s
choice.
 DR SLEDGE: One of the practical implications of this discussion is that it is
almost impossible to sort all this out in any clinically reasonable time frame
during a patient encounter. It would be wonderful to have strategies to facilitate
this because there’s no way that anybody in the community has enough time for these kinds of conversations with the average patient.
DR SLEDGE: One of the practical implications of this discussion is that it is
almost impossible to sort all this out in any clinically reasonable time frame
during a patient encounter. It would be wonderful to have strategies to facilitate
this because there’s no way that anybody in the community has enough time for these kinds of conversations with the average patient.
Select publications

