
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2-3
![]() DR LOVE: What are your thoughts on the US Oncology trial data
comparing adjuvant AC to TC, reported by Steve Jones?
DR LOVE: What are your thoughts on the US Oncology trial data
comparing adjuvant AC to TC, reported by Steve Jones?
![]() DR SWAIN: I have a lot of confidence in docetaxel. I’ve used it for a long
time, and my patients have done well. I’ve used the 100-mg/m2 dose alone
quite often, so I don’t have a problem using 75 mg/m2 with cyclophosphamide.
DR SWAIN: I have a lot of confidence in docetaxel. I’ve used it for a long
time, and my patients have done well. I’ve used the 100-mg/m2 dose alone
quite often, so I don’t have a problem using 75 mg/m2 with cyclophosphamide.
In addition, the TC regimen brings no cardiac toxicity and probably less risk of leukemia because you don’t use an anthracycline or growth factors. I recently read an article in the Journal of Clinical Oncology examining the risk of leukemia for patients treated for breast cancer, so that’s been on my mind (Le Deley 2007). We probably won’t see that with TC, and that’s great because we certainly don’t want these patients who are at low risk — or any patients, for that matter — developing leukemia.
Track 6
![]() DR LOVE: Can you discuss the design and goals of the NSABP-B-40
neoadjuvant trial?
DR LOVE: Can you discuss the design and goals of the NSABP-B-40
neoadjuvant trial?
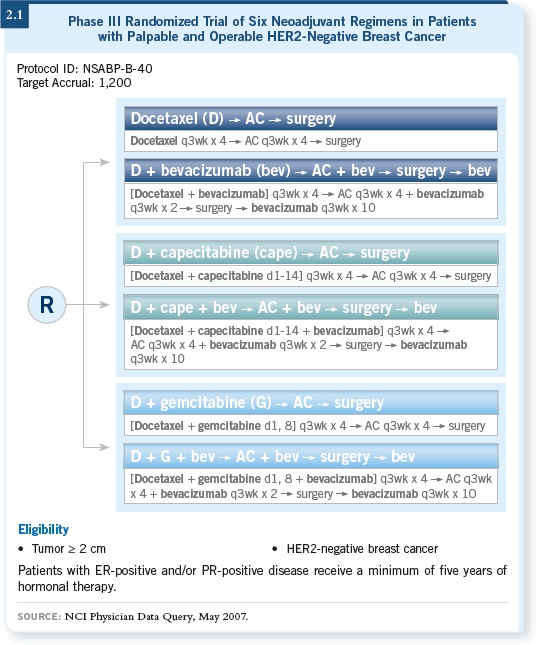
![]() DR SWAIN: In this study, patients are randomly assigned to receive docetaxel
versus docetaxel/capecitabine versus docetaxel/gemcitabine, and all three
agents are followed by AC (2.1). In addition, each arm is further randomized
to bevacizumab pre- and postoperatively or no bevacizumab.
DR SWAIN: In this study, patients are randomly assigned to receive docetaxel
versus docetaxel/capecitabine versus docetaxel/gemcitabine, and all three
agents are followed by AC (2.1). In addition, each arm is further randomized
to bevacizumab pre- and postoperatively or no bevacizumab.
One of the primary goals of the trial is to evaluate whether a baseline gene or microarray analysis, performed before the patients receive any treatment, will predict which patients will respond. We’re moving more into the twenty-first century, trying to tailor treatment and see if we can predict response. In addition, we’re evaluating biologic therapy with the bevacizumab randomization.
Track 7
![]() DR LOVE: Can you discuss the paper your group recently published in the
Journal of Clinical Oncology examining the anti-angiogenic and antitumor
effects of bevacizumab in women with inflammatory breast cancer?
DR LOVE: Can you discuss the paper your group recently published in the
Journal of Clinical Oncology examining the anti-angiogenic and antitumor
effects of bevacizumab in women with inflammatory breast cancer?
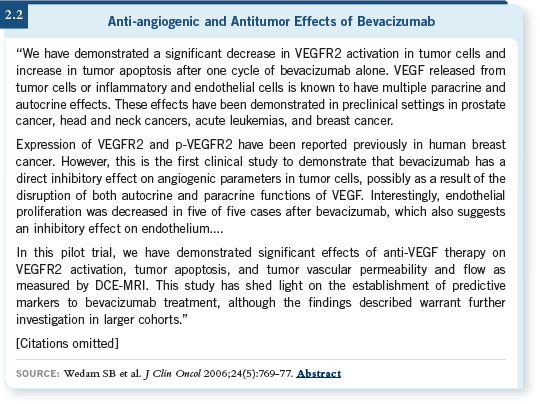
![]() DR SWAIN: We conducted a small pilot study in which we administered
bevacizumab alone for one cycle, followed by bevacizumab and chemotherapy,
to patients with inflammatory breast cancer (Wedam 2006; [2.2]). The goal
was to identify molecular markers that would predict response to bevacizumab,
so biopsies were performed before and after the bevacizumab monotherapy.
DR SWAIN: We conducted a small pilot study in which we administered
bevacizumab alone for one cycle, followed by bevacizumab and chemotherapy,
to patients with inflammatory breast cancer (Wedam 2006; [2.2]). The goal
was to identify molecular markers that would predict response to bevacizumab,
so biopsies were performed before and after the bevacizumab monotherapy.
We found consistent decrease in the activated VEGFR-2 in the tumor cells in patients treated with bevacizumab alone. This is the receptor through which VEGF-A acts to increase angiogenesis. We also found a decrease in vascular permeability and flow by MRI.
Another interesting finding was an increase in apoptosis with bevacizumab alone, similar to that seen by Chang with trastuzumab (Mohsin 2005). We didn’t find any correlation to response because it was a small pilot study and almost all the patients responded. That was great for the patients, but we didn’t have a large dichotomy to examine.
The next step will be to examine the effects of bevacizumab on the activated receptor and apoptosis in larger studies. Dennis Slamon is conducting a similar kind of study evaluating TAC with or without bevacizumab, and we hope he will examine those issues.
![]() DR LOVE: I was struck by the fact that bevacizumab appears to be working
against the tumor cells directly. Did that surprise you?
DR LOVE: I was struck by the fact that bevacizumab appears to be working
against the tumor cells directly. Did that surprise you?
![]() DR SWAIN: When we designed this study, Ferrara had written reviews and
conducted a lot of research geared toward evaluating these receptors and
VEGF acting on the endothelial cells (Ferrara 1992, 1997). Although a couple
of papers had been published showing that the VEGF receptors were present
on tumor cells, it wasn’t felt to be a significant mechanism of action.
DR SWAIN: When we designed this study, Ferrara had written reviews and
conducted a lot of research geared toward evaluating these receptors and
VEGF acting on the endothelial cells (Ferrara 1992, 1997). Although a couple
of papers had been published showing that the VEGF receptors were present
on tumor cells, it wasn’t felt to be a significant mechanism of action.
However, I’m more convinced now that it’s really the tumor cells that are important, and I do believe that’s the key finding of this study. The stroma is important as well, especially in inflammatory cancer, but that’s an aside.
Track 10-11
![]() DR LOVE: Where are we with regard to the next generation of adjuvant
trials of women with HER2-positive tumors? I understand that the
BCIRG and NSABP are considering a study of TCH with or without
bevacizumab.
DR LOVE: Where are we with regard to the next generation of adjuvant
trials of women with HER2-positive tumors? I understand that the
BCIRG and NSABP are considering a study of TCH with or without
bevacizumab.
![]() DR SWAIN: We have had many heated discussions about the design of this
study, but based on the second interim analysis of the BCIRG 006 trial,
which showed TCH was similar in efficacy to AC
DR SWAIN: We have had many heated discussions about the design of this
study, but based on the second interim analysis of the BCIRG 006 trial,
which showed TCH was similar in efficacy to AC![]() TH, people are much
more comfortable now with omitting the anthracycline (Slamon 2006).
However, some groups in Europe will have an additional arm consisting of
AC
TH, people are much
more comfortable now with omitting the anthracycline (Slamon 2006).
However, some groups in Europe will have an additional arm consisting of
AC![]() TH with or without bevacizumab for those physicians who still feel
they need to use an anthracycline.
TH with or without bevacizumab for those physicians who still feel
they need to use an anthracycline.
![]() DR LOVE: In your practice, how do you treat patients with node-positive,
HER2-positive disease?
DR LOVE: In your practice, how do you treat patients with node-positive,
HER2-positive disease?
![]() DR SWAIN: Based on the BCIRG 006 data, I feel comfortable using TCH
with those patients (Slamon 2006).
DR SWAIN: Based on the BCIRG 006 data, I feel comfortable using TCH
with those patients (Slamon 2006).
![]() DR LOVE: Do you think we’ll get to the point that anthracyclines will no
longer be used in adjuvant therapy of all breast cancers?
DR LOVE: Do you think we’ll get to the point that anthracyclines will no
longer be used in adjuvant therapy of all breast cancers?
![]() DR SWAIN: I hope that’s the case because I believe that anthracycline-associated
cardiotoxicity is an important issue, and I believe clinicians will be
moving away from anthracyclines in the future.
DR SWAIN: I hope that’s the case because I believe that anthracycline-associated
cardiotoxicity is an important issue, and I believe clinicians will be
moving away from anthracyclines in the future.
![]() DR LOVE: What is a patient’s risk of developing a serious cardiac problem or
leukemia with TCH?
DR LOVE: What is a patient’s risk of developing a serious cardiac problem or
leukemia with TCH?
![]() DR SWAIN: The risk of cardiac toxicity is low — almost zero. We don’t see
cardiac toxicity with trastuzumab monotherapy, and, although some clots and
arrhythmias were reported on the TCH arm, the rate of heart failure is just
about zero.
DR SWAIN: The risk of cardiac toxicity is low — almost zero. We don’t see
cardiac toxicity with trastuzumab monotherapy, and, although some clots and
arrhythmias were reported on the TCH arm, the rate of heart failure is just
about zero.
As for leukemia, I expect you’ll see less on the TCH arm, but the numbers are small at this point.
Track 13
![]() DR LOVE: You recently wrote an editorial in the JCO regarding the
Oncotype DX assay and the NSABP-B-20 data (Paik 2006). Can you
comment on that (Swain 2006)?
DR LOVE: You recently wrote an editorial in the JCO regarding the
Oncotype DX assay and the NSABP-B-20 data (Paik 2006). Can you
comment on that (Swain 2006)?
![]() DR SWAIN: In the NSABP-B-20 study, patients with ER-positive, node-negative
disease were randomly assigned to tamoxifen with or without chemotherapy.
DR SWAIN: In the NSABP-B-20 study, patients with ER-positive, node-negative
disease were randomly assigned to tamoxifen with or without chemotherapy.
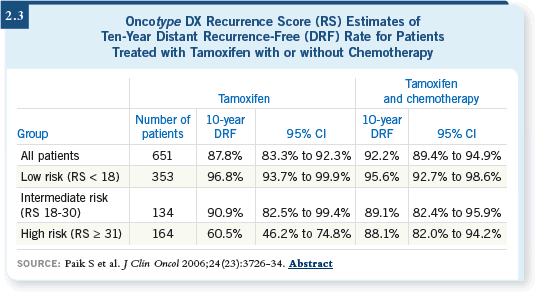
The trial showed a benefit with the addition of chemotherapy, and when the Oncotype DX assay was performed, investigators found that patients with a high recurrence score benefited from chemotherapy, whereas those with a low or intermediate recurrence score did not (Paik 2006; [2.3]).
To me, this is an outstanding contribution. I now use Oncotype DX for all my patients who have ER-positive, HER2-negative, node-negative breast cancer, and I’ve found it to be helpful.
![]() DR LOVE: Do you rely on Oncotype DX for patients with larger tumors? For
example, would you be comfortable omitting chemotherapy for a patient with
node-negative disease but a 4-cm tumor if her recurrence score was low?
DR LOVE: Do you rely on Oncotype DX for patients with larger tumors? For
example, would you be comfortable omitting chemotherapy for a patient with
node-negative disease but a 4-cm tumor if her recurrence score was low?
![]() DR SWAIN: Absolutely. I believe that it’s all about the biology and not the
size, particularly.
DR SWAIN: Absolutely. I believe that it’s all about the biology and not the
size, particularly.
The other important benefit of Oncotype DX, which was presented at the San Antonio Breast Cancer Symposium, is its reliability in measuring the estrogen receptor (Kim 2006). I believe RT-PCR may be the best way to measure the estrogen receptor. We know immunohistochemistry (IHC) is fraught with problems, and I’m uncomfortable when I receive an estrogen receptor assay result from a small laboratory.