
 |
||||||||

| Tracks 1-15 | ||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you comment on the US Oncology adjuvant trial
comparing TC (docetaxel/cyclophosphamide) to AC chemotherapy?
DR LOVE: Would you comment on the US Oncology adjuvant trial
comparing TC (docetaxel/cyclophosphamide) to AC chemotherapy?
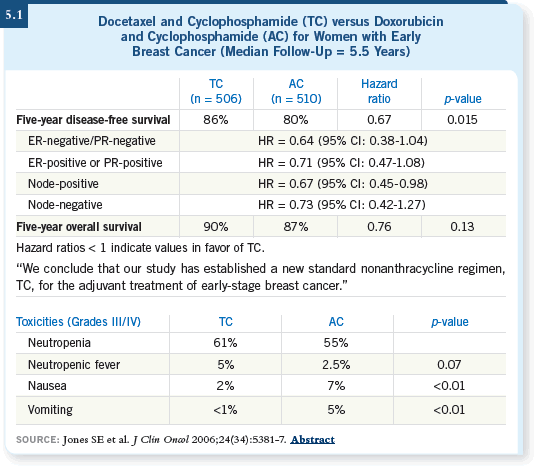
![]() DR O’REGAN: It is somewhat small for an adjuvant study, but it is useful in
that it provides data for both node-positive and node-negative disease ( Jones
2006). The data demonstrated that disease-free survival is better with TC.
DR O’REGAN: It is somewhat small for an adjuvant study, but it is useful in
that it provides data for both node-positive and node-negative disease ( Jones
2006). The data demonstrated that disease-free survival is better with TC.
In my practice, I find the 100-mg/m2 dose of docetaxel as a single agent difficult to administer, but the 75-mg/m2 regimen with TC is relatively easy, so it does beg the question of whether you need AC. However, most patients do well with AC, and it is a tried-and-tested chemotherapy regimen.
![]() DR LOVE: Initially, when Steve Jones presented this at San Antonio (5.1),
some people questioned the reported improved tolerability of TC. Do you see
this improvement as the result of the lower docetaxel dose?
DR LOVE: Initially, when Steve Jones presented this at San Antonio (5.1),
some people questioned the reported improved tolerability of TC. Do you see
this improvement as the result of the lower docetaxel dose?
![]() DR O’REGAN: Yes. Also, patients don’t experience nausea with the lower dose,
which is nice.
DR O’REGAN: Yes. Also, patients don’t experience nausea with the lower dose,
which is nice.
Track 5-7
![]() DR LOVE: What’s your take on nab paclitaxel and its clinical utility?
DR LOVE: What’s your take on nab paclitaxel and its clinical utility?
![]() DR O’REGAN: I am using nab paclitaxel, and I’m surprised that it hasn’t been
used more extensively, particularly in the first-line setting. Many clinicians are
still using it for patients who fail at least one taxane, whereas the first-line data
are pretty robust, and nab paclitaxel’s effectiveness and toxicity profile are better
than those of regular paclitaxel. So I am using it in the first-line setting, where
I tend to use the every three-week schedule, and perhaps weekly later on.
DR O’REGAN: I am using nab paclitaxel, and I’m surprised that it hasn’t been
used more extensively, particularly in the first-line setting. Many clinicians are
still using it for patients who fail at least one taxane, whereas the first-line data
are pretty robust, and nab paclitaxel’s effectiveness and toxicity profile are better
than those of regular paclitaxel. So I am using it in the first-line setting, where
I tend to use the every three-week schedule, and perhaps weekly later on.
Although this was a randomized Phase II trial, Bill Gradishar’s data demonstrated that the response rate with the weekly schedule of nab paclitaxel was double that of docetaxel and it was considerably less toxic (Gradishar 2006).
The weekly schedule of nab paclitaxel also appeared to cause less neuropathy. Neuropathy with this agent is an issue, but a lot of patients I’ve treated have received prior taxanes, so I’m not sure how it will perform in the first-line setting in that regard. In my experience, nab paclitaxel is well tolerated.
![]() DR LOVE: Some people also believe that the neuropathy resolves more quickly
with nab paclitaxel. What’s your impression?
DR LOVE: Some people also believe that the neuropathy resolves more quickly
with nab paclitaxel. What’s your impression?
![]() DR O’REGAN: I have not been able to “tweak that out” in my own patients.
The data are weak because the pivotal trial (Gradishar 2005) included only
five patients in the first-line paclitaxel arm.
DR O’REGAN: I have not been able to “tweak that out” in my own patients.
The data are weak because the pivotal trial (Gradishar 2005) included only
five patients in the first-line paclitaxel arm.
The nab paclitaxel arm in this trial had 24. It is possible because of the way nab paclitaxel works that the neuropathy may resolve more quickly, but I’d like to see more data.
![]() DR LOVE: How useful is it clinically to have a shorter infusion time and no
need for premedication?
DR LOVE: How useful is it clinically to have a shorter infusion time and no
need for premedication?
![]() DR O’REGAN: That’s a huge advantage, particularly the lack of premedication.
Patients complain about having to take the steroids with paclitaxel. In terms of
the shorter infusion time, that is a huge benefit in a busy practice.
DR O’REGAN: That’s a huge advantage, particularly the lack of premedication.
Patients complain about having to take the steroids with paclitaxel. In terms of
the shorter infusion time, that is a huge benefit in a busy practice.
Track 11
![]() DR LOVE: What are your thoughts about bevacizumab? Are you using it
off study?
DR LOVE: What are your thoughts about bevacizumab? Are you using it
off study?
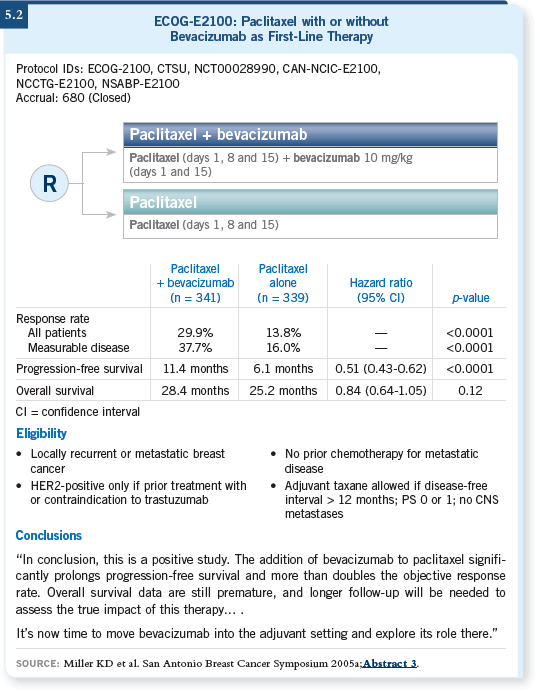
![]() DR O’REGAN: Yes. When you consider the ECOG-E2100 trial (Miller 2005a),
the progression-free survival increase of nearly six months is impressive (5.2).
Certainly this benefit may be compared to the combination versus single-agent
chemotherapy studies we’ve conducted before in metastatic breast cancer.
In my experience, bevacizumab works well.
DR O’REGAN: Yes. When you consider the ECOG-E2100 trial (Miller 2005a),
the progression-free survival increase of nearly six months is impressive (5.2).
Certainly this benefit may be compared to the combination versus single-agent
chemotherapy studies we’ve conducted before in metastatic breast cancer.
In my experience, bevacizumab works well.
Most of the patients I’ve treated have shown at least some type of a response, although perhaps not as sustained as we would like. I also like bevacizumab because its toxicity doesn’t overlap with that of the chemotherapy. Apart from a little hypertension and some headaches, patients tolerate it well.
![]() DR LOVE: When you’ve used bevacizumab, has it been only in the first-line
setting or also in the second line or beyond?
DR LOVE: When you’ve used bevacizumab, has it been only in the first-line
setting or also in the second line or beyond?
![]() DR O’REGAN: I’ve used it almost exclusively in the first-line setting with paclitaxel.
This is one area in which I believe nab paclitaxel is being used in practice.
I have seen some patients from the community who’ve been receiving nab paclitaxel and bevacizumab. For a couple of patients, I’ve used it outside of the
first-line setting, but as you would expect, we do not obtain many responses.
DR O’REGAN: I’ve used it almost exclusively in the first-line setting with paclitaxel.
This is one area in which I believe nab paclitaxel is being used in practice.
I have seen some patients from the community who’ve been receiving nab paclitaxel and bevacizumab. For a couple of patients, I’ve used it outside of the
first-line setting, but as you would expect, we do not obtain many responses.
I do wonder whether bevacizumab should be considered for other patients in addition to those with metastatic disease, such as those with locally recurrent cancer. It would be interesting to see whether they’re more sensitive to the bevacizumab, because some of those patients are difficult to treat.
![]() DR LOVE: What are your thoughts on using other chemotherapeutic agents,
such as capecitabine, with bevacizumab?
DR LOVE: What are your thoughts on using other chemotherapeutic agents,
such as capecitabine, with bevacizumab?
![]() DR O’REGAN: It would probably work out fine to administer it with
capecitabine. Unfortunately, we have a somewhat negative trial in the second-line
setting with bevacizumab and capecitabine, although a response-rate
improvement was evident in that trial (Miller 2005b). I believe it’s the line of
therapy used rather than the agent you use it with that’s important.
DR O’REGAN: It would probably work out fine to administer it with
capecitabine. Unfortunately, we have a somewhat negative trial in the second-line
setting with bevacizumab and capecitabine, although a response-rate
improvement was evident in that trial (Miller 2005b). I believe it’s the line of
therapy used rather than the agent you use it with that’s important.
At San Antonio, an NCCTG trial of docetaxel/capecitabine with bevacizumab was presented (Perez 2006). This regimen showed activity, although it was somewhat toxic. Emerging data suggest that bevacizumab is effective with chemotherapy agents other than paclitaxel, and I have on at least one occasion used capecitabine and bevacizumab for a patient who was not a candidate for paclitaxel in the first-line setting.
![]() DR LOVE: When you use bevacizumab with a chemotherapeutic agent, do
you continue the therapy until progression?
DR LOVE: When you use bevacizumab with a chemotherapeutic agent, do
you continue the therapy until progression?
![]() DR O’REGAN: If a patient continues to respond, I continue both agents until
disease progression, as was done on the trial.
DR O’REGAN: If a patient continues to respond, I continue both agents until
disease progression, as was done on the trial.
Of course, the big question is whether you could drop the chemotherapy and continue the bevacizumab, but I haven’t done that. In some ways, it may make more sense to continue the bevacizumab on its own, but that must be addressed in a trial.