|
||||||||

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: How do you approach endocrine therapy for postmenopausal
patients with receptor-positive disease?
DR LOVE: How do you approach endocrine therapy for postmenopausal
patients with receptor-positive disease?
![]() DR PRITCHARD: It’s clear now that in the adjuvant endocrine setting, every
postmenopausal woman should receive an aromatase inhibitor at some point.
I tend to start most of my patients on an aromatase inhibitor up front.
DR PRITCHARD: It’s clear now that in the adjuvant endocrine setting, every
postmenopausal woman should receive an aromatase inhibitor at some point.
I tend to start most of my patients on an aromatase inhibitor up front.
It’s clear that adding an aromatase inhibitor at the end of five years of tamoxifen, or after two to three years of tamoxifen, is additionally beneficial. In the last few years it’s come down the pipeline that every one of the three different aromatase inhibitors seems to be useful in each setting.
We’re still awaiting data that might tell us whether it’s as good or even better to start with a year or two of tamoxifen — whether there’s priming from tamoxifen — and then to switch over to an aromatase inhibitor. Also, what do you do for the patient who has received five years of an aromatase inhibitor? Is that too long, just right or not long enough?
Track 2
![]() DR LOVE: How are you approaching patients who are completing five
years of an aromatase inhibitor?
DR LOVE: How are you approaching patients who are completing five
years of an aromatase inhibitor?
![]() DR PRITCHARD: It’s arbitrary that we studied five years of aromatase inhibitors.
The data we have from MA17 and other studies have been clear that
continuing to administer letrozole brings additional benefit year after year, at
least in the post-tamoxifen setting (Ingle 2006).
DR PRITCHARD: It’s arbitrary that we studied five years of aromatase inhibitors.
The data we have from MA17 and other studies have been clear that
continuing to administer letrozole brings additional benefit year after year, at
least in the post-tamoxifen setting (Ingle 2006).
It seems to be steady up to four years of follow-up, and it may be good administered indefinitely. Right now, we haven’t studied more than five years, so I would stop the drug or place patients in a clinical trial at the end of five years of an aromatase inhibitor.
![]() DR LOVE: Do you discuss the option of continuing?
DR LOVE: Do you discuss the option of continuing?
![]() DR PRITCHARD: Yes, and currently in Canada, neither of the trials that will
evaluate patients in that setting — NSABP-B-42 or the rerandomization of
MA17 — are available.
DR PRITCHARD: Yes, and currently in Canada, neither of the trials that will
evaluate patients in that setting — NSABP-B-42 or the rerandomization of
MA17 — are available.
We should be able to enroll patients on them in the next three to six months. Until then, I’ve been administering an aromatase inhibitor a little longer and hoping to place these patients on a trial.
NSABP-B-42 will randomly assign women who have undergone any endocrine therapy that adds up to five years and includes an aromatase inhibitor to either five more years of letrozole or not.
The rerandomization of MA17 randomly assigns all women who received five years of tamoxifen and five years of an aromatase inhibitor as part of the MA17 study to receive more letrozole or not.
Now we have an amendment, which will randomly assign anyone who has received five years of an aromatase inhibitor to more aromatase inhibitor or not. So to some degree, it will overlap with NSABP-B-42 but not completely.
Track 4
![]() DR LOVE: What do we know about the etiology and management of the
arthralgias that are sometimes seen with the aromatase inhibitors?
DR LOVE: What do we know about the etiology and management of the
arthralgias that are sometimes seen with the aromatase inhibitors?
![]() DR PRITCHARD: I don’t believe we understand it well. Most commonly we
see a syndrome of aches and pains. It’s an arthralgia/myalgia syndrome but not
arthritis. In most of the controlled studies, it’s only about five or 10 percent of
patients.
DR PRITCHARD: I don’t believe we understand it well. Most commonly we
see a syndrome of aches and pains. It’s an arthralgia/myalgia syndrome but not
arthritis. In most of the controlled studies, it’s only about five or 10 percent of
patients.
I believe it’s a real syndrome. I suspect it’s similar to the aches and pains some women describe around menopause and that it’s an estrogen-deprivation symptom of some type. Some experience it, some don’t seem to at all and a few people experience severe symptoms. I try taking them off and switching them to another aromatase inhibitor or switching them back to tamoxifen. In some patients, it seems to dissipate — maybe as a result of the switch.
Track 6
![]() DR LOVE: How do you manage treatment for postmenopausal women
who are a few years out after receiving tamoxifen?
DR LOVE: How do you manage treatment for postmenopausal women
who are a few years out after receiving tamoxifen?
![]() DR PRITCHARD: It appears that the risk of relapse for patients with endocrine-responsive
breast cancer is steady and goes on for 10 or 15 years. For patients
with node-positive disease, it may be as much as four percent a year, and
for people with node-negative disease, it may be around two percent a year.
When you add that up over five or 10 years, it’s substantial.
DR PRITCHARD: It appears that the risk of relapse for patients with endocrine-responsive
breast cancer is steady and goes on for 10 or 15 years. For patients
with node-positive disease, it may be as much as four percent a year, and
for people with node-negative disease, it may be around two percent a year.
When you add that up over five or 10 years, it’s substantial.
If I see patients out at that time point, I approach them about receiving an aromatase inhibitor in the postmenopausal setting. The data are good, certainly in MA17 (Ingle 2006). When the study was closed early, women who had been receiving a placebo for three or more years were offered the opportunity to receive letrozole. The women who went on letrozole benefited. It’s not a randomized comparison, but it appears as though starting letrozole later still provided benefit.
Track 7
![]() DR LOVE: Where are we in terms of aromatase inhibitors and bone health?
DR LOVE: Where are we in terms of aromatase inhibitors and bone health?
![]() DR PRITCHARD: All of the aromatase inhibitors are associated with osteoporosis
and increased fracture rates. So I believe these women should be
monitored, have baseline bone mineral density (BMD) recorded, take calcium
and vitamin D, exercise and receive bisphosphonates if it’s appropriate.
DR PRITCHARD: All of the aromatase inhibitors are associated with osteoporosis
and increased fracture rates. So I believe these women should be
monitored, have baseline bone mineral density (BMD) recorded, take calcium
and vitamin D, exercise and receive bisphosphonates if it’s appropriate.
A few trials show that you can prevent most of the osteoporosis and most of the BMD loss with bisphosphonates (Brufsky 2007; Gnant 2007). However, I don’t believe that is clear, even in the long-term follow-up of bisphosphonates in healthy women (Bone 2004).
We’re also beginning to see some reports of bisphosphonate-associated jaw necrosis. Will that be a long-term side effect that becomes increasingly common over time? I would like to believe that if we treated these patients proactively, we could prevent most of the osteoporosis.
Track 9
![]() DR LOVE: Can you discuss Bill Gradishar’s presentation at San Antonio of
the EFECT results and your thoughts about those data?
DR LOVE: Can you discuss Bill Gradishar’s presentation at San Antonio of
the EFECT results and your thoughts about those data?
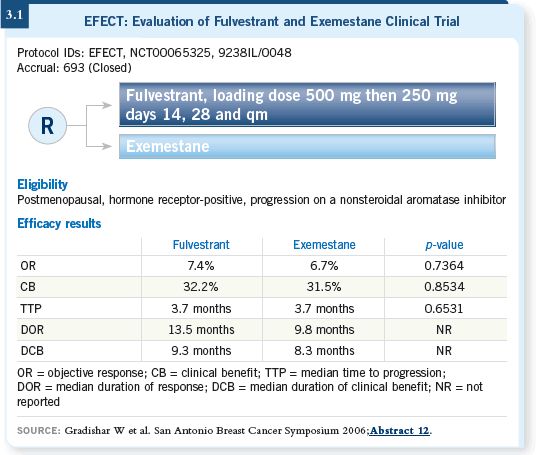
![]() DR PRITCHARD: The EFECT study compared exemestane to fulvestrant
for patients whose disease progressed on a nonsteroidal aromatase inhibitor
(Gradishar 2006; [3.1]). I would have guessed that fulvestrant would be more
active in that setting because it’s a drug of a totally different class.
DR PRITCHARD: The EFECT study compared exemestane to fulvestrant
for patients whose disease progressed on a nonsteroidal aromatase inhibitor
(Gradishar 2006; [3.1]). I would have guessed that fulvestrant would be more
active in that setting because it’s a drug of a totally different class.
However, lots of data have suggested that exemestane, as a steroidal aromatase inhibitor, provides decent response rates or a decent level of clinical benefit beyond the use of nonsteroidal aromatase inhibitors. So it was perhaps a surprise, and perhaps not, that exemestane and fulvestrant in that setting appear almost identical. In fact, even the side-effect profiles are similar.
![]() DR LOVE: In EFECT, they used a loading dose of fulvestrant, which more
people are using now in practice. What are your thoughts on that?
DR LOVE: In EFECT, they used a loading dose of fulvestrant, which more
people are using now in practice. What are your thoughts on that?
![]() DR PRITCHARD: Good pharmacokinetic data indicate that using no loading
dose, it takes three to four months to reach steady-state levels. I believe most
of us are using a loading dose, and there doesn’t seem to be any real problem
with that. A number of trials have been using the loading dose, and no safety
problems are apparent.
DR PRITCHARD: Good pharmacokinetic data indicate that using no loading
dose, it takes three to four months to reach steady-state levels. I believe most
of us are using a loading dose, and there doesn’t seem to be any real problem
with that. A number of trials have been using the loading dose, and no safety
problems are apparent.
Track 10
![]() DR LOVE: What’s your algorithm for sequential hormonal therapy in the
metastatic setting, both for the premenopausal and the postmenopausal
patient?
DR LOVE: What’s your algorithm for sequential hormonal therapy in the
metastatic setting, both for the premenopausal and the postmenopausal
patient?
![]() DR PRITCHARD: In the premenopausal setting, I discuss tamoxifen or ovarian
ablation. For me, ovarian ablation would involve starting the patient on a
luteinizing hormone-releasing hormone analog and then ordering a surgical
oophorectomy if she is willing to undergo that. I also usually discuss the
option of receiving both ovarian ablation and tamoxifen.
DR PRITCHARD: In the premenopausal setting, I discuss tamoxifen or ovarian
ablation. For me, ovarian ablation would involve starting the patient on a
luteinizing hormone-releasing hormone analog and then ordering a surgical
oophorectomy if she is willing to undergo that. I also usually discuss the
option of receiving both ovarian ablation and tamoxifen.
Once the patient has a permanent ovarian ablation, I generally administer an aromatase inhibitor and fulvestrant. Ongoing studies are evaluating fulvestrant in premenopausal patients.
In the postmenopausal setting, I would use an aromatase inhibitor first — unless the disease has progressed on an aromatase inhibitor, which is becoming more common — followed by tamoxifen followed by fulvestrant. We are conducting a study in our center right now evaluating two doses of fulvestrant after progression on an aromatase inhibitor. I believe one could use these hormones in almost any order in the metastatic setting.
Track 19
![]() DR LOVE: Would you discuss the TAnDEM trial data of patients with
ER- and HER2-positive, metastatic disease?
DR LOVE: Would you discuss the TAnDEM trial data of patients with
ER- and HER2-positive, metastatic disease?
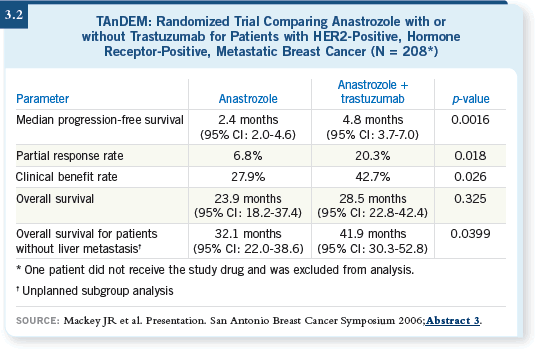
![]() DR PRITCHARD: The TAnDEM trial was the first trial to evaluate trastuzumab
in combination with an endocrine agent and compared anastrozole/
trastuzumab to anastrozole alone (Mackey 2006; [3.2]). The aromatase inhibitor/
trastuzumab arm had considerably longer progression-free survival, but
the median progression-free survival in both arms was short — between two
and three months versus between four and five months.
DR PRITCHARD: The TAnDEM trial was the first trial to evaluate trastuzumab
in combination with an endocrine agent and compared anastrozole/
trastuzumab to anastrozole alone (Mackey 2006; [3.2]). The aromatase inhibitor/
trastuzumab arm had considerably longer progression-free survival, but
the median progression-free survival in both arms was short — between two
and three months versus between four and five months.
The stunning aspect is that this is a group of women who don’t generally respond well to endocrine therapy, even with the addition of trastuzumab. But within that median progression-free survival, some patients go into long responses or periods of stability with anastrozole and trastuzumab, and there may even be some patients who are able to do that with anastrozole alone.
I believe the reasonable approach for patients like that — if they’re relatively asymptomatic and you can monitor them closely — is trying an endocrine agent with trastuzumab or an endocrine agent alone. If you treat them with an endocrine agent alone and they have visceral disease, you have to watch them closely and monitor liver function, et cetera, to make sure no disease or symptom becomes unmanageable.
You could also make the argument that, on average, this is a group of patients who don’t respond well to endocrine treatment with or without trastuzumab, and perhaps you should move ahead to something more energetic, such as a taxane with trastuzumab.