
 |
||||||||
| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss what we currently understand about predictive
tissue markers of response to aromatase inhibitors?
DR LOVE: Can you discuss what we currently understand about predictive
tissue markers of response to aromatase inhibitors?
![]() DR PEREZ: Some important translational data have been released over the
past year, and these data have implications for practice. One implication is
that there is no point in paying too much attention to progesterone receptor
status as it relates to the benefit of aromatase inhibitors. Confusion was raised when the initial data from ATAC were reported based on local testing for
ER and PR. Based on central testing for ER and PR, the trans-ATAC data
presented at the 2006 San Antonio meeting demonstrated that patients benefit
from anastrozole regardless of whether the PR status is positive or negative
(Dowsett 2006). These data are consistent with the data from the other aromatase
inhibitor studies (Viale 2005). Those data should help clinicians be confident
about using aromatase inhibitors independent of the PR status, as long as
the ER status is positive.
DR PEREZ: Some important translational data have been released over the
past year, and these data have implications for practice. One implication is
that there is no point in paying too much attention to progesterone receptor
status as it relates to the benefit of aromatase inhibitors. Confusion was raised when the initial data from ATAC were reported based on local testing for
ER and PR. Based on central testing for ER and PR, the trans-ATAC data
presented at the 2006 San Antonio meeting demonstrated that patients benefit
from anastrozole regardless of whether the PR status is positive or negative
(Dowsett 2006). These data are consistent with the data from the other aromatase
inhibitor studies (Viale 2005). Those data should help clinicians be confident
about using aromatase inhibitors independent of the PR status, as long as
the ER status is positive.
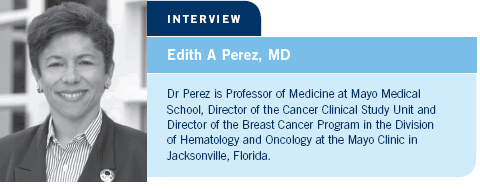
Another issue, also related to the testing and use of aromatase inhibitors, is HER2 status. Many people believed that aromatase inhibitors worked only for HER2-positive disease, but now we have data demonstrating that aromatase inhibitors benefit patients with HER2-positive and HER2-negative disease (Dowsett 2006; [1.1]).
Track 3
![]() DR LOVE: Can you discuss the study you’re conducting that evaluates
vinorelbine, capecitabine and trastuzumab?
DR LOVE: Can you discuss the study you’re conducting that evaluates
vinorelbine, capecitabine and trastuzumab?
![]() DR PEREZ: We know both drugs — vinorelbine and capecitabine — show
activity in refractory breast cancer as well as up front. Preclinically, both
drugs have been shown to work with trastuzumab. Initial data regarding the
potential interaction of capecitabine with trastuzumab confused some people
(Pegram 2004).
DR PEREZ: We know both drugs — vinorelbine and capecitabine — show
activity in refractory breast cancer as well as up front. Preclinically, both
drugs have been shown to work with trastuzumab. Initial data regarding the
potential interaction of capecitabine with trastuzumab confused some people
(Pegram 2004).
However, elegant data from a group in China showed us nice response rates associated with that combination (Xu 2006). We also know that the combination of capecitabine and vinorelbine can be well tolerated and associated with good responses (Welt 2005). Patients can expect a good quality of life and no alopecia.
Based on this information, we decided to combine all of these data and create a triple-drug regimen comprising capecitabine, vinorelbine and trastuzumab. Patients who received trastuzumab as adjuvant or first-line therapy for metastatic disease are permitted into the study. We did not require prior taxane therapy. We’ve almost completed accrual to this study, and so far, the tolerability is excellent.
![]() DR LOVE: Do you use the vinorelbine/capecitabine combination for HER 2-negative tumors ?
DR LOVE: Do you use the vinorelbine/capecitabine combination for HER 2-negative tumors ?
![]() DR PEREZ: Sometimes we do because it has good activity and tolerability.
DR PEREZ: Sometimes we do because it has good activity and tolerability.
Track 6-7
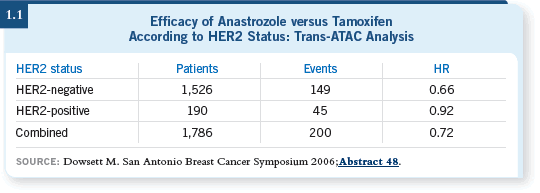
![]() DR LOVE: Can you summarize your thoughts about the BCIRG 006
presentation by Dennis Slamon in San Antonio (1.2)?
DR LOVE: Can you summarize your thoughts about the BCIRG 006
presentation by Dennis Slamon in San Antonio (1.2)?
![]() DR PEREZ: As a result of these data, the TCH regimen is now an alternative
to AC followed by a taxane/trastuzumab (TH). Statistically speaking,
no differences exist between AC
DR PEREZ: As a result of these data, the TCH regimen is now an alternative
to AC followed by a taxane/trastuzumab (TH). Statistically speaking,
no differences exist between AC![]() TH and the TCH regimen as reported
(Slamon 2006).
TH and the TCH regimen as reported
(Slamon 2006).
However, a caveat in the numbers might be of interest. Comparing TCH and
AC![]() TH, the AC
TH, the AC![]() TH group reported 16 additional cases of congestive
heart failure, but the TCH group reported 14 additional breast cancer events,
including breast cancer relapse and death.
TH group reported 16 additional cases of congestive
heart failure, but the TCH group reported 14 additional breast cancer events,
including breast cancer relapse and death.
![]() DR LOVE: When you see a patient with a HER2-positive, node-positive
tumor who is otherwise healthy, what chemotherapy regimen do you generally
utilize?
DR LOVE: When you see a patient with a HER2-positive, node-positive
tumor who is otherwise healthy, what chemotherapy regimen do you generally
utilize?
![]() DR PEREZ: We use AC
DR PEREZ: We use AC ![]() T (paclitaxel) H as our standard approach — the
regimen approved by the FDA. However, we’re considering additional steps
these days in our practice.
T (paclitaxel) H as our standard approach — the
regimen approved by the FDA. However, we’re considering additional steps
these days in our practice.
One example is the use of ACE inhibitors to potentially decrease the risk of cardiac toxicity associated with both anthracyclines and, potentially, trastuzumab. As medical oncologists, we haven’t been well attuned to the potential value of ACE inhibitors for improving cardiovascular risk in illnesses besides hypertension or those associated with underlying cardiac disease.
These agents might be helpful by reducing the cardiac afterload, which may have clinical implications. We have data indicating that ACE inhibitors may decrease the risk of anthracycline-related cardiac toxicity. We need to address the use of ACE inhibitors in cancer therapy, so we are proposing to conduct a trial. I believe this practice is worthy of investigation, and we will pursue it vigorously during the next few months.
Track 9
![]() DR LOVE: What is your clinical approach for patients with smaller,
HER2-positive, node-negative tumors?
DR LOVE: What is your clinical approach for patients with smaller,
HER2-positive, node-negative tumors?
![]() DR PEREZ: In the clinical trials, few patients had tumors smaller than one
centimeter, so we lack solid data related to the absolute improvement of
adding trastuzumab in that setting.
DR PEREZ: In the clinical trials, few patients had tumors smaller than one
centimeter, so we lack solid data related to the absolute improvement of
adding trastuzumab in that setting.
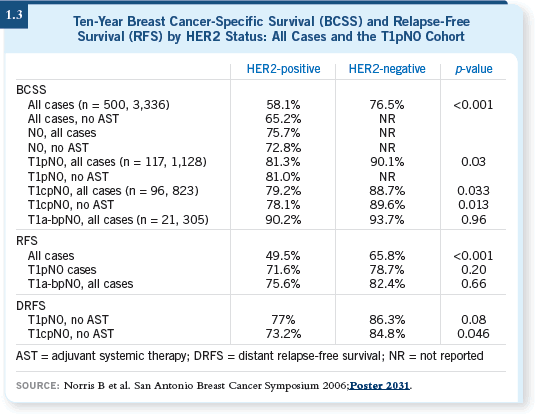
However, two intriguing presentations at the 2006 San Antonio meeting addressed the natural history of small, HER2-positive breast tumors, demonstrating that these patients appeared to have a poor prognosis (Black 2006; Norris 2006; [1.3]).
My interpretation of the data is that HER2 status is the driver of prognosis more than tumor size. So I tend to be more open to discussing the potential of using adjuvant trastuzumab for patients with small breast tumors, although I am clearly pointing out that the eligibility criteria of the studies did not include them.
We’re making that recommendation outside of the FDA boundaries because they have only approved adjuvant trastuzumab for patients with node-positive breast cancer. From what I understand, that was based on the data from the joint analysis (NSABP-B-31 and NCCTG-N9831) because only six percent of the patients had node-negative disease.
However, as practicing oncologists, we know data from other studies that enrolled large numbers of patients with node-negative disease demonstrated a benefit in adding trastuzumab (Piccart-Gebhart 2005; Slamon 2006; Smith 2007).
So I have zero caveats related to the recommendation of using adjuvant trastuzumab according to nodal status. I prefer to overtreat rather than undertreat because undertreatment may lead to patient death.
![]() DR LOVE: Despite a lack of data, we do have a long history with the concept
of relative risk reduction in the adjuvant therapy of breast cancer. It seems as if
this can be applied to HER2-positive tumors and trastuzumab.
DR LOVE: Despite a lack of data, we do have a long history with the concept
of relative risk reduction in the adjuvant therapy of breast cancer. It seems as if
this can be applied to HER2-positive tumors and trastuzumab.
![]() DR PEREZ: I completely agree. The relative benefits appear to be the same,
irrespective of the subgroup analyzed. So the matter at hand will be to understand
the natural history of small, HER2-positive breast tumors and whether
HER2 testing has been done correctly.
DR PEREZ: I completely agree. The relative benefits appear to be the same,
irrespective of the subgroup analyzed. So the matter at hand will be to understand
the natural history of small, HER2-positive breast tumors and whether
HER2 testing has been done correctly.
Data from presentations at San Antonio and also from a manuscript published by Steve Chia in the Journal of Clinical Oncology (Chia 2004) demonstrate that patients with small tumors — even those smaller than one centimeter — that are Grade III have a 10-year relapse rate that appears to exceed 25 percent.
That is the figure I’m showing to my patients — a relapse rate of about 25 percent at five to 10 years. Based on all the information we have, that kind of relapse risk warrants adjuvant systemic therapy.
Track 17-18
![]() DR LOVE: Where do you see nab paclitaxel fitting into the treatment of
breast cancer?
DR LOVE: Where do you see nab paclitaxel fitting into the treatment of
breast cancer?
![]() DR PEREZ: The data with every three-week nab paclitaxel versus once every
three-week paclitaxel (Gradishar 2005) justify the FDA approval. Data from
the randomized Phase II trial, which evaluated nab paclitaxel or docetaxel
once every three weeks, are tantalizing (Gradishar 2006). I’m happy that there
will be a formal, randomized Phase III trial of nab paclitaxel weekly versus
docetaxel once every three weeks.
DR PEREZ: The data with every three-week nab paclitaxel versus once every
three-week paclitaxel (Gradishar 2005) justify the FDA approval. Data from
the randomized Phase II trial, which evaluated nab paclitaxel or docetaxel
once every three weeks, are tantalizing (Gradishar 2006). I’m happy that there
will be a formal, randomized Phase III trial of nab paclitaxel weekly versus
docetaxel once every three weeks.
![]() DR LOVE: What about the issue of nab paclitaxel in combination with trastuzumab
for the patient with HER2-positive disease?
DR LOVE: What about the issue of nab paclitaxel in combination with trastuzumab
for the patient with HER2-positive disease?
![]() DR PEREZ: The combination clearly shows activity, and an ongoing Phase II
study from Memorial is evaluating nab paclitaxel/carboplatin and trastuzumab.
The benefit of this drug is that it doesn’t require premedication. So even if the
nab paclitaxel were similar in efficacy to paclitaxel, I would still tend to favor
it for the potential impact on the quality of life of my patients.
DR PEREZ: The combination clearly shows activity, and an ongoing Phase II
study from Memorial is evaluating nab paclitaxel/carboplatin and trastuzumab.
The benefit of this drug is that it doesn’t require premedication. So even if the
nab paclitaxel were similar in efficacy to paclitaxel, I would still tend to favor
it for the potential impact on the quality of life of my patients.
![]() DR LOVE: Can you discuss the study comparing nab paclitaxel to docetaxel
(Gradishar 2006; [1.4])?
DR LOVE: Can you discuss the study comparing nab paclitaxel to docetaxel
(Gradishar 2006; [1.4])?
![]() DR PEREZ: This was a 300-patient, randomized Phase II study with four
arms. In three of the arms, the patients received nab paclitaxel, and the fourth
group received docetaxel.
DR PEREZ: This was a 300-patient, randomized Phase II study with four
arms. In three of the arms, the patients received nab paclitaxel, and the fourth
group received docetaxel.
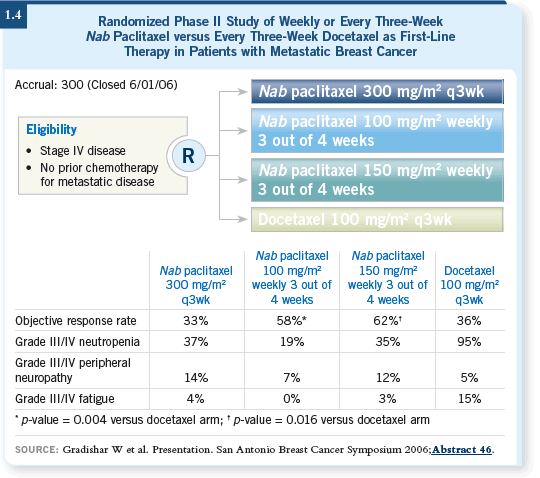
The three nab paclitaxel arms received 300 mg/m2 once every three weeks or one of two weekly regimens, either 100 or 150 mg/m2 three weeks out of four. The docetaxel arm received 100 mg/m2 of docetaxel once every three weeks.
The response data were fascinating: 33 percent for the once every three-week nab paclitaxel and 36 percent for the once every three-week docetaxel (1.4), which is what I would expect from a single-agent taxane in the first-line treatment of metastatic disease. However, the responses for the two weekly nab paclitaxel arms were about 58 and 62 percent — beautiful response rates for these weekly regimens. That’s why investigators will use 100 mg/m2 weekly as the appropriate comparator against docetaxel for the Phase III study.
This randomized Phase II study was telling, not only because of the response rate — the progression-free survival rates are still premature, although the numbers are starting to look interesting — but also because the tolerability was good for the weekly nab paclitaxel, 100 mg/m2 three weeks out four.