
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1-3
![]() DR LOVE: Can you review the MINDACT trial?
DR LOVE: Can you review the MINDACT trial?
![]() DR PICCART-GEBHART: This study (BIG 3-04) has a design similar to
TAILORx. The 6,000 women enrolled will be assessed by the 70-gene signature
MammaPrint assay and Adjuvant! Online (www.AdjuvantOnline.com),
which we believe is one of the best tools available to predict outcomes for
women with breast cancer.
DR PICCART-GEBHART: This study (BIG 3-04) has a design similar to
TAILORx. The 6,000 women enrolled will be assessed by the 70-gene signature
MammaPrint assay and Adjuvant! Online (www.AdjuvantOnline.com),
which we believe is one of the best tools available to predict outcomes for
women with breast cancer.
If both tools indicate a high risk of recurrence, the woman will receive adjuvant chemotherapy. If both tools indicate a low risk of recurrence, the patient will not receive chemotherapy. Most of these women will be treated with adjuvant endocrine therapy.
The discordant group, in which the two tools do not provide the same information, is the critical group. These patients will be randomly assigned to either trusting Adjuvant! Online and ignoring the 70-gene signature or trusting the signature and ignoring the clinical and pathological factors integrated in Adjuvant! Online. We want to prove that the 70-gene signature is right. It is interesting that in 80 percent of these discordant cases, the signature indicates that the patient has low-risk disease, whereas Adjuvant! Online tells you she has a high risk of recurrence.
![]() DR LOVE: This assay requires fresh tissue, correct?
DR LOVE: This assay requires fresh tissue, correct?
![]() DR PICCART-GEBHART: Yes, which is a big challenge.
DR PICCART-GEBHART: Yes, which is a big challenge.
![]() DR LOVE: Why is it that you decided to pursue this assay in the face of the
Oncotype DX assay, which doesn’t require fresh tissue and seems to have more
data behind it at the moment?
DR LOVE: Why is it that you decided to pursue this assay in the face of the
Oncotype DX assay, which doesn’t require fresh tissue and seems to have more
data behind it at the moment?
![]() DR PICCART-GEBHART: You are right — in terms of validation, Oncotype DX
is farther down the road (Paik 2004, 2006), but we believe that in 10 years we
will have even better signatures. By using the microarray technology, in fact,
we will obtain information on the full genome for all 6,000 women. So we
will observe not only the 70 genes but also the entire genome.
DR PICCART-GEBHART: You are right — in terms of validation, Oncotype DX
is farther down the road (Paik 2004, 2006), but we believe that in 10 years we
will have even better signatures. By using the microarray technology, in fact,
we will obtain information on the full genome for all 6,000 women. So we
will observe not only the 70 genes but also the entire genome.
That represents a fabulous source of data for translational research because it will allow us to analyze potentially different signatures that will be developed in the next five years — for example, signatures for predicting organ-site metastases.
![]() DR LOVE: Is the MammaPrint assay currently being used in clinical practice?
DR LOVE: Is the MammaPrint assay currently being used in clinical practice?
![]() DR PICCART-GEBHART: No, not that I am aware of. I strongly believe that
prospective validation is essential. However, it is FDA approved and commercially
available. I suspect some oncologists may want to use it for select cases in
which they have doubts and the traditional factors are in the gray area.
DR PICCART-GEBHART: No, not that I am aware of. I strongly believe that
prospective validation is essential. However, it is FDA approved and commercially
available. I suspect some oncologists may want to use it for select cases in
which they have doubts and the traditional factors are in the gray area.
Track 5
![]() DR LOVE: Let’s talk about adjuvant treatment of patients with HER2-
positive tumors. In your own practice outside of a clinical trial setting,
how are you approaching patients with node-positive disease?
DR LOVE: Let’s talk about adjuvant treatment of patients with HER2-
positive tumors. In your own practice outside of a clinical trial setting,
how are you approaching patients with node-positive disease?
![]() DR PICCART-GEBHART: I ask myself two questions: Am I worried about an
early relapse, and am I worried about the risk of cardiac toxicity?
DR PICCART-GEBHART: I ask myself two questions: Am I worried about an
early relapse, and am I worried about the risk of cardiac toxicity?
We conducted an interesting analysis in the HERA study observing the patterns of relapse according to hormone receptor status and nodal status in women on the control arm who did not receive trastuzumab. Women with four or more positive nodes had a high risk of relapse in the first two years, which indicates that these women are probably better served with a strategy that uses trastuzumab up front.
We also discovered that women with hormone receptor-negative tumors have a higher risk of early relapse compared to those with hormone receptor-positive tumors. Therefore, I use that information to decide between immediate or delayed trastuzumab.
We need to do a lot more work in terms of identifying cardiac risk factors. Some early signals have been provided by the analysis of cardiac toxicity in the NSABP (Tan-Chiu 2005) and HERA studies (Piccart-Gebhart 2005; Smith 2007). In the presence of these risk factors, I will think twice about the use of anthracyclines and perhaps favor a regimen like TCH.
Track 6-8
![]() DR LOVE: Would you discuss the ALTTO trial?
DR LOVE: Would you discuss the ALTTO trial?
![]() DR PICCART-GEBHART: This study will accrue approximately 8,000 women
(4.1). We wanted to investigate several possible approaches using chemotherapy
and several anti-HER2 treatments, including lapatinib or trastuzumab or the
sequence or combination of trastuzumab and lapatinib.
DR PICCART-GEBHART: This study will accrue approximately 8,000 women
(4.1). We wanted to investigate several possible approaches using chemotherapy
and several anti-HER2 treatments, including lapatinib or trastuzumab or the
sequence or combination of trastuzumab and lapatinib.
The combination of trastuzumab and lapatinib everyone understands because you can achieve maximal inhibition by attacking the receptors on both sides. That should be the better arm, but the sequential strategy is also important in case the toxicities associated with the combination are problematic.
![]() DR LOVE: The sequential arm will use trastuzumab first followed by a rest
period and then lapatinib?
DR LOVE: The sequential arm will use trastuzumab first followed by a rest
period and then lapatinib?
![]() DR PICCART-GEBHART: Yes. We have more data with this type of sequence.
For practical reasons, we also felt it would be difficult for the patients to start
with an oral drug and then after a few months go back to the hospital to
receive an intravenous treatment.
DR PICCART-GEBHART: Yes. We have more data with this type of sequence.
For practical reasons, we also felt it would be difficult for the patients to start
with an oral drug and then after a few months go back to the hospital to
receive an intravenous treatment.
![]() DR LOVE: Which chemotherapy regimens are allowed on the trial?
DR LOVE: Which chemotherapy regimens are allowed on the trial?
![]() DR PICCART-GEBHART: This is a worldwide effort, and no standard chemotherapy
regimen is accepted throughout the world. Also, we wanted to offer
this trial to countries where taxanes are still problematic. So we are not
requiring taxanes, but we believe the vast majority of women will receive
anthracyclines and taxanes.
DR PICCART-GEBHART: This is a worldwide effort, and no standard chemotherapy
regimen is accepted throughout the world. Also, we wanted to offer
this trial to countries where taxanes are still problematic. So we are not
requiring taxanes, but we believe the vast majority of women will receive
anthracyclines and taxanes.
At the beginning of the trial, the taxanes will be limited to weekly paclitaxel administered concomitantly with the anti-HER2 treatment for safety reasons. We have safety data for paclitaxel in combination with lapatinib, trastuzumab and the combination. We do not have those data for docetaxel or nab paclitaxel, but we hope to obtain the safety data. If these look good, we will amend the protocol and these other taxanes will also be allowed.
The trial will have two strata (4.1). For physicians and patients who choose the strategy of chemotherapy combined with the anti-HER2 therapy, anthracycline- based chemotherapy will be administered first. The selection of a chemotherapy regimen will be open and flexible.
For example, it may be four cycles of AC or three cycles of FEC. That is followed by weekly paclitaxel combined with the anti-HER2 treatment.
In the second group, physicians will be able to choose from a list of candidate regimens. They will have to administer the chemotherapy according to the HERA philosophy, by which the chemotherapy is administered first and then patients are randomly assigned to receive biologic therapy, which is not administered in combination with chemotherapy.
![]() DR LOVE: What are the eligibility criteria for the study?
DR LOVE: What are the eligibility criteria for the study?
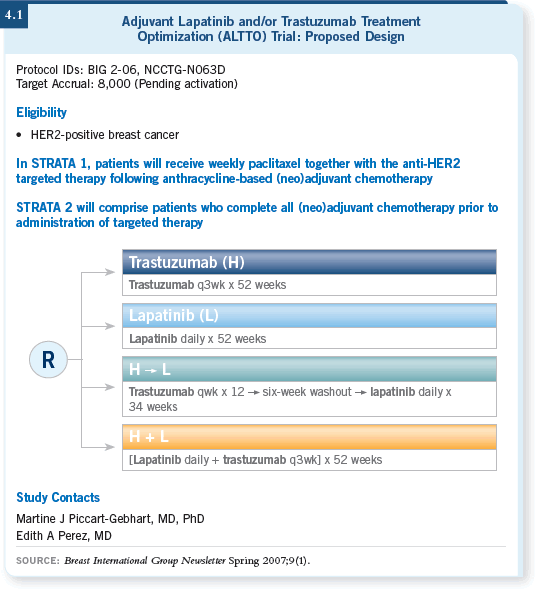
![]() DR PICCART-GEBHART: The trial is for women up to age 70 with a tumor that
is one centimeter or greater in size. A difference in comparison to the previous
adjuvant trastuzumab trials is that we will define HER2 positivity according to
the recently published ASCO guidelines (Wolff 2007). Positivity means IHC
staining of 3+, but you now need more than 30 percent of the cells stained for
the tumor to be considered 3+. FISH positivity is also defined slightly differently
than before in that the FISH ratio has to be greater than 2.2 (Wolff 2007).
DR PICCART-GEBHART: The trial is for women up to age 70 with a tumor that
is one centimeter or greater in size. A difference in comparison to the previous
adjuvant trastuzumab trials is that we will define HER2 positivity according to
the recently published ASCO guidelines (Wolff 2007). Positivity means IHC
staining of 3+, but you now need more than 30 percent of the cells stained for
the tumor to be considered 3+. FISH positivity is also defined slightly differently
than before in that the FISH ratio has to be greater than 2.2 (Wolff 2007).
Track 9
![]() DR LOVE: How do you approach patients with smaller, HER2-positive,
node-negative disease in a clinical setting, particularly those with tumors
smaller than one centimeter?
DR LOVE: How do you approach patients with smaller, HER2-positive,
node-negative disease in a clinical setting, particularly those with tumors
smaller than one centimeter?
![]() DR PICCART-GEBHART: It’s a big dilemma. We don’t have a clear answer, and
we have to struggle with the decision about whether to use trastuzumab. At
my hospital, we have finally decided to use a cutoff of six millimeters. We
ignore the possibility of using trastuzumab if the tumor is smaller than that.
When the tumor is six millimeters or greater, particularly if the patient is a
young woman with an ER-negative tumor, we offer trastuzumab.
DR PICCART-GEBHART: It’s a big dilemma. We don’t have a clear answer, and
we have to struggle with the decision about whether to use trastuzumab. At
my hospital, we have finally decided to use a cutoff of six millimeters. We
ignore the possibility of using trastuzumab if the tumor is smaller than that.
When the tumor is six millimeters or greater, particularly if the patient is a
young woman with an ER-negative tumor, we offer trastuzumab.
Track 15
![]() DR LOVE: What are your thoughts about long-term management of
women with ER-positive breast cancer?
DR LOVE: What are your thoughts about long-term management of
women with ER-positive breast cancer?
![]() DR PICCART-GEBHART: We became aware that hormone receptor-positive
breast cancer is a strange disease with a continuous risk of relapse over time.
This is bad news because this disease might well be extremely difficult to
cure. If that’s the case, we need to consider long-term endocrine therapy.
DR PICCART-GEBHART: We became aware that hormone receptor-positive
breast cancer is a strange disease with a continuous risk of relapse over time.
This is bad news because this disease might well be extremely difficult to
cure. If that’s the case, we need to consider long-term endocrine therapy.
I hope that with pharmacogenomic tools we will be able to identify patients at continued risk of relapse and those who can be cured with a relatively short course of endocrine treatment. I believe the majority will need prolonged endocrine manipulation. We don’t know the optimal sequencing for that.
Currently in my practice, I try to use eight years of hormonal therapy. It’s not based on solid evidence — rather, it’s a compromise drawn from the data with five years of tamoxifen, five years of an aromatase inhibitor, and two to three years of tamoxifen followed by two to three years of an aromatase inhibitor. I try to administer two to three years of tamoxifen and five years of an aromatase inhibitor.
I’m waiting impatiently for the results from BIG 1-98 because one arm involves an aromatase inhibitor followed by tamoxifen. Those results should be available in 2008.
![]() DR LOVE: If a woman has completed five years of an aromatase inhibitor, are
there situations in which you’ll continue it?
DR LOVE: If a woman has completed five years of an aromatase inhibitor, are
there situations in which you’ll continue it?
![]() DR PICCART-GEBHART: Yes, if the woman is at particularly high risk with
many positive nodes and has been tolerating the therapy well. Of course, I will
first do a careful evaluation of the status of her bones, lipids and quality of life.
DR PICCART-GEBHART: Yes, if the woman is at particularly high risk with
many positive nodes and has been tolerating the therapy well. Of course, I will
first do a careful evaluation of the status of her bones, lipids and quality of life.