Debu Tripathy, MD |
EDITED COMMENTS |
 Editor’s note: Editor’s note:
This interview was conducted shortly before the release of findings of NSABP-B-31, NCCTG- 9831 and the HERA trial, all evaluating trastuzumab in the adjuvant setting.
Biologic rationale for continuing trastuzumab after disease progression
The rationale is purely speculative. We know trastuzumab and chemotherapy work synergistically in the laboratory. In the clinic, they are at least additive. One of the questions is whether that synergy might exist with another chemotherapeutic agent, maybe through another mechanism.
When a patient’s disease becomes resistant to trastuzumab plus a taxane, there may still be some synergy between trastuzumab and another chemotherapeutic agent. This is one of the main biologic reasons to consider continuing trastuzumab, which is different from the usual paradigm in cancer treatment in which we don’t ever treat patients with any drug that has been associated with clinical resistance.
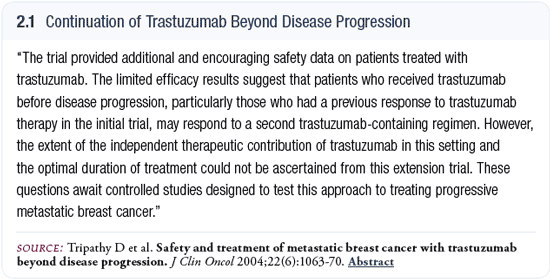
Second-line response data from trastuzumab pivotal trial
I published a follow-up report (Tripathy 2004) of the crossover portion from the pivotal trastuzumab study (2.1). In the pivotal trial, patients with metastatic disease were randomly assigned to chemotherapy alone or chemotherapy plus trastuzumab, and they were allowed to cross over to trastuzumab upon progression. Even the patients who were receiving chemotherapy plus trastuzumab could cross over to trastuzumab with another chemotherapy. A few patients received trastuzumab in combination with hormonal therapy (Slamon 2001).
It’s important to recognize that this was an expanded-access trial. It did not require scans at regular times, and it didn’t have the rigorous follow-up of a regular clinical trial. We looked at the data retrospectively and had a very conservative estimate of the response rate. If patients did not have data from a scan or from physical exams, we would not classify them as responders (Tripathy 2004).
In the group of patients who were initially treated with trastuzumab plus chemotherapy, we found that 11 percent had an objective response when they received trastuzumab beyond progression. In the group of patients who initially received chemotherapy alone and crossed over to trastuzumab with or without chemotherapy, the response rate was 14 percent (Tripathy 2004), which is similar to the results reported by Cobleigh in patients who had been previously treated with chemotherapy (Cobleigh 1999).
This expanded-access trial demonstrated that there is some activity with the continuation of trastuzumab, and we didn’t see any safety issues (Tripathy 2004). But the trial doesn’t tell us about the independent contribution of trastuzumab in this situation. This will only be answered with trials that randomly assign patients who are progressing on trastuzumab to chemotherapy alone or chemotherapy plus trastuzumab.
Nonprotocol approach to patients whose disease progresses on a trastuzumab-containing regimen
Right now, in the absence of data, we have to use our best knowledge and extensions of the clinical and laboratory data to guide our patients. I personally believe there may be a role for continuing trastuzumab with another chemotherapeutic agent. My patients also usually feel strongly about it, and we often elect to go that route. I don’t use this approach with all of my patients, and I certainly explain to them that we don’t know the answer.
In this situation, trastuzumab appears to be safe. The rate of cardiotoxicity on the extension trial was very low in the patients who were already on trastuzumab and hadn’t developed cardiac problems (Tripathy 2004). I generally continue trastuzumab, but not indefinitely. Once a patient goes through two or three combinations, I think it’s probably prudent to stop trastuzumab and try either single-agent or combination chemotherapy.
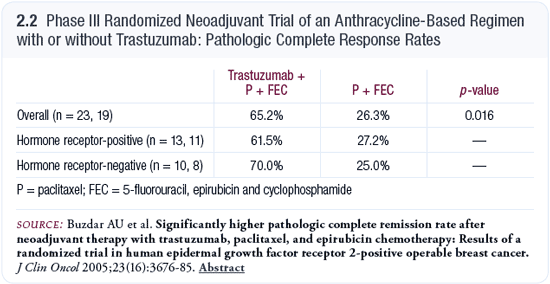
MD Anderson Phase III randomized neoadjuvant trial of an anthracycline-based regimen with or without trastuzumab
Many of us would have guessed that the pathologic complete response (pCR) rate would be high. However, we were all surprised when we saw the magnitude of difference for the neoadjuvant trastuzumab regimen, which had a pCR rate in the 60 percent range (Buzdar 2005; [2.2]). We had never seen pCR rates so high. Obviously, this needs to be validated in a larger study, and one is planned.
A potential explanation for such a high pCR rate is that the patients received longer duration chemotherapy (paclitaxel and FEC) instead of just four cycles. Another reason might be that synergy exists between the anthracyclines and trastuzumab, which has not been previously tested because of the concerns of cardiotoxicity. They did report some subclinical reductions in ejection fractions in the patients on the trastuzumab arm, but not much in the way of clinical cardiotoxicity (Buzdar 2005).
Role of adjuvant aromatase inhibitors
I believe a clear, consistent story is emerging without a lot of conflicts and conundrums — adjuvant aromatase inhibitors are better than tamoxifen. Whether the aromatase inhibitors are used at the time of initial diagnosis, after two to three years or five years of tamoxifen, there is a favorable impact on local, distant and even contralateral breast cancer recurrences.
The unresolved questions are: Should you use a little tamoxifen, maybe two years, and then cross over? Should you just use an aromatase inhibitor right off the bat and maybe even think of continuing beyond five years? The trial that will provide the most information in this regard is the BIG FEMTA/BIG 1-98 trial, which is comparing: (1) five years of letrozole, (2) five years of tamoxifen, (3) two years of letrozole followed by three years of tamoxifen and (4) two years of tamoxifen followed by three years of letrozole.
The results from the noncrossover arms have already been reported at the 2005 St Gallen Conference (Thürlimann 2005; Kudachadkar 2005). At 26 months of follow-up, there is the expected benefit, very similar to what was seen in the ATAC trial. The length of follow-up for the BIG FEMTA trial is nowhere near the length of follow-up in the ATAC trial, but the hazard ratios seem to be in the same neighborhood. We obviously need more follow-up time. The data on the crossover arms, which are of greatest interest, have not been reported.
Management of postmenopausal women who have completed five years of an adjuvant aromatase inhibitor
At this point, I discontinue the adjuvant aromatase inhibitor after the completion of five years of therapy. This is an area where you would discuss things with the patient. It reminds me of the situation I used to have with tamoxifen 10 years ago. I used to leave patients on adjuvant tamoxifen longer. They would be uncomfortable coming off, and we didn’t have any data at that point. Since that time, we have had data from at least one study, NSABP-B-14, in which rerandomization to a total of 10 years of adjuvant tamoxifen showed an actual increase in relapse compared to five years of adjuvant tamoxifen (Fisher 2001).
We have to be very careful with this. Obviously, bone mineral density is one issue. It looks as though, from both the Austrian study (Gnant 2004) and the Zometa-Femara Adjuvant Synergy Trial (Z-FAST; [Brufsky 2004]), that early intervention with a bisphosphonate can essentially abrogate the loss in bone mineral density. However, there are other symptoms of total estrogen deprivation that we may not know about yet (eg, effects on the vascular or CNS system). I believe exposing a patient to more than five years of an adjuvant aromatase inhibitor at this point involves uncharted waters in terms of risks.
Management of postmenopausal women in the midst of receiving five years of adjuvant tamoxifen
With the data I have now, my recommendation is to switch those patients to an aromatase inhibitor. I won’t call them and have them rush to the clinic, but when I see them next, I will review the data and switch over at whatever point in the course of therapy they are, whether it’s at one, two, three or four years. It’s hard for me to say, “Let’s just leave you on tamoxifen for another two years,” because in the crossover studies, the effect on recurrences seems to occur soon after changing therapy. I believe at any point, a woman is better off with aromatase inhibitors. The big question is: Is there any way to recapture some of the benefit with tamoxifen on the back end?
Management of postmenopausal women who have completed five years of adjuvant tamoxifen
In this situation, my opinion might be different from what you’ve heard before. Patients who are off of tamoxifen still have a risk of recurrence, and one can extrapolate the benefits of an aromatase inhibitor to right after the patient discontinues tamoxifen or sometime later. I think we need to estimate the patient’s residual risk. We know that at five years there’s still a considerable risk, especially among patients at high risk. Once you go out to 10 and 15 years, then the risks all start to converge, but they’re still around 0.5 to one percent per year.
Over a five- to 10-year period, that risk could add up to seven to 10 percent. If you can reduce the risk by one third, then it might be worth it. I actually believe it’s reasonable to consider aromatase inhibitors in any patient with hormone receptor-positive breast cancer who is within a 10- or even 15-year period. It may sound like a big departure from what others are saying, but based on the clinical and biological data, I believe it’s a reasonable thing to do. The caveat, again, is that we have to monitor for side effects.
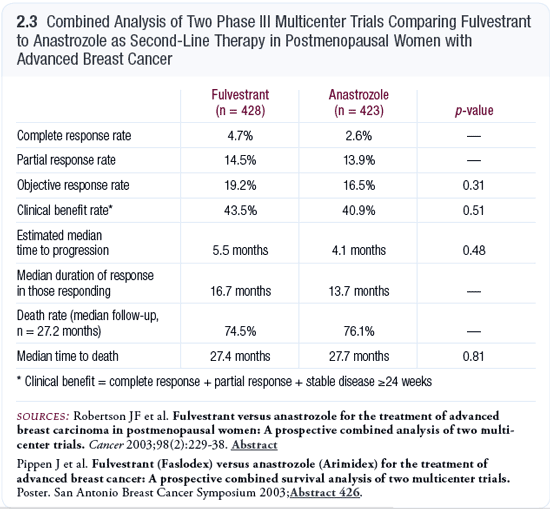
Role of fulvestrant
The trials of fulvestrant conducted to date do not provide a clear indication as to where we should be using this drug. The up-front study comparing tamoxifen to fulvestrant was essentially equivalent. As second-line therapy, fulvestrant seemed to perform equally as well as anastrozole (Robertson 2003; [2.3]).
At this point in time, the sequencing and timing for fulvestrant are unclear. I think it’s reasonable to use the drug — maybe not up front, but as second- or third-line therapy. This is where you might look at the patient’s preferences in terms of an intramuscular or an oral drug.
A recent study of 261 women with metastatic breast cancer demonstrated that about one third preferred a monthly intramuscular injection (Paley 2005). I would have guessed that 10 percent or less of the women would prefer an intramuscular injection. I’ve always assumed that oral drugs were preferable, if they were equally effective. Therefore, I was surprised to see that many patients preferred an intramuscular injection. I need to query my patients more when I start looking at these options.
Chemotherapy selection in patients with metastatic disease
When we have many chemotherapy drugs that, as single agents, provide response rates that overlap with each other, it shouldn’t be looked at as a conundrum, but rather as an opportunity to individualize therapy based on the sideeffect profiles. I’m starting to use drugs with less toxicity first, because we generally see the longest duration of response with the drug we use first. We might as well have that long period of time be the one with the least toxicity. Utilizing an agent that does not result in hair loss should be considered, if that’s important to the patient. Or, in the patient with pre-existing neuropathy from diabetes or prior chemotherapy, avoidance of an agent with neurotoxicity is important.
For me, the single most important factor is what treatment the patient has previously received. If a patient has progressed on an adjuvant taxane, I’m more likely to use a nontaxane drug. Although, granted, you can see responses to docetaxel and nanoparticle albumin-bound (nab) paclitaxel upon progression with the original paclitaxel formulation.
Nab paclitaxel
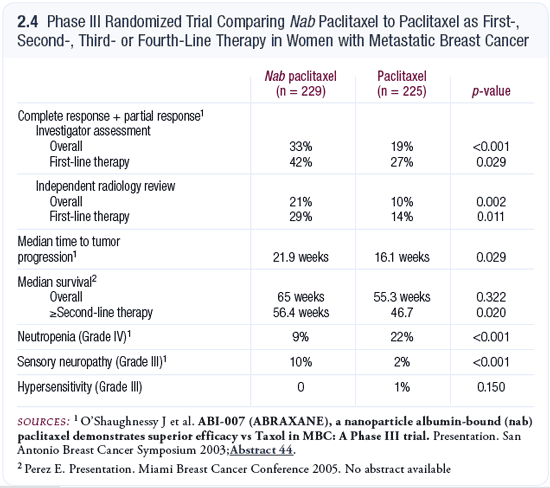
The availability of nab paclitaxel is a welcome advance in drug delivery. Combining paclitaxel tightly with a nanoparticle allows it to dissolve without the use of Cremophor®, which is one of the compounds in the original paclitaxel formulation that causes acute allergic reactions and necessitates the use of steroids. Evidence also exists from laboratory models that you may have better tumor penetration with nab paclitaxel.
What is happening in humans is hard to know, but in a head-to-head study, the clinical endpoints of response rate and time to progression were actually improved with nab paclitaxel compared to the original paclitaxel formulation. It was a difficult comparison because the doses weren’t the same.
It may be that nab paclitaxel was more tolerable, and patients were able to receive a higher dose; therefore, they had a better response. When we look at most of the toxicities, however, there were fewer with nab paclitaxel. The exception was peripheral neuropathy, for which nab paclitaxel had a higher incidence (O’Shaughnessy 2003; [2.4]). This may have been related to the overall dose of paclitaxel.
Choice of taxanes in the metastatic setting
A weekly regimen of the original paclitaxel formulation would have been my choice in the past. Now that we have data with nab paclitaxel, I think that’s a reasonable option also. From the data, nab paclitaxel may be preferable.
It outperformed the original paclitaxel formulation when administered every three weeks (O’Shaughnessy 2003). A weekly regimen also seems to outperform an every three-week regimen of the original paclitaxel formulation (Seidman 2004), and I’m left wondering which is the best drug to use.
For patients who prefer an every three-week schedule, I believe nab paclitaxel is the way to go. Otherwise, it’s a toss-up between every three-week nab paclitaxel and a weekly regimen of the original paclitaxel formulation. I don’t believe there’s a way to compare the two. CALGB is planning to conduct a head-to-head trial comparing weekly regimens of nab paclitaxel and the original paclitaxel formulation.
First-line therapy for patients with metastatic disease who received adjuvant AC and a taxane
I look at these patients as being anthracycline and taxane refractory, but if a long period has passed (ie, two or more years) since the adjuvant therapy, you could certainly retry a taxane. Nab paclitaxel or a weekly regimen of the original paclitaxel formulation would be attractive choices. However, I’m generally treating these patients as anthracycline and taxane refractory, and I’m using capecitabine. Not only is capecitabine FDA approved for that indication, it seems to have among the higher response rates in the anthracycline- and taxane-refractory group of patients.
Alternatives to capecitabine would include vinorelbine and gemcitabine. I believe combinations of these drugs are also something to consider. We’re so geared toward thinking of single agents, but combinations do have a role, particularly for more symptomatic patients. It’s hard to know which combination wins out. Data exist on combinations of vinorelbine/capecitabine, gemcitabine/ vinorelbine and gemcitabine/capecitabine.
Select publications
 |
Dr Tripathy is a Professor of Internal Medicine and Director of the Komen UT Southwestern Breast Cancer Research Program at the University of Texas Southwestern Medical Center in Dallas, Texas. |
|

