|
On a recent muggy Miami morning, I motored up I-95 to visit the breast cancer
practice of medical oncologist Sandra Franco, my former colleague at the
University of Miami Sylvester Cancer Center. Our CME group recently received
a grant from the National Cancer Institute through the Small Business Innovative
Research mechanism to produce and evaluate a pilot DVD to assist in patient
education regarding clinical trials. As part of this effort, Sandra had arranged
for us to videotape interviews with four of her patients who are participating in
current NSABP randomized studies. This visit was highly enlightening.
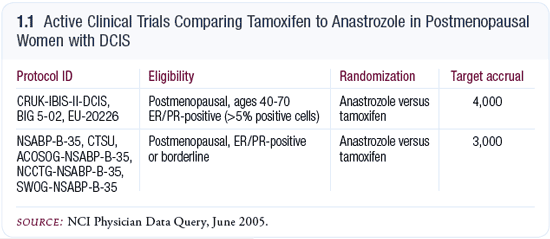
The DVD is focused specifically on NSABP-B-35, which randomly assigns
postmenopausal women with ER-positive DCIS to either tamoxifen or anastrozole.
Like many other NSABP trials in breast and colon cancer, this study addresses a
simple but important clinical question and is likely to provide a clear answer. In
the United Kingdom, the IBIS-II trial has an identical randomization (1.1).
The constantly mushrooming database on the use of adjuvant aromatase inhibitors
would seemingly make the results of B-35 easy to predict. (Women on
anastrozole will experience fewer recurrences, second primary breast cancers,
endometrial cancers, hysterectomies and strokes, but more arthralgias and
potentially more fractures, depending on how meticulously docs follow bone
densities and intervene when necessary.) However, the age of evidence-based
oncology means that many physicians and patients want definitive Phase III data
before moving AIs from invasive to noninvasive disease, and B-35 will likely
deliver the goods, albeit a few years from now.
On the DVD, Richard Margolese, the principal investigator of B-35, reviews the
background and rationale for the study and what he tells his patients about the
potential risks and benefits of participation. Sandra Franco and her oncology
research nurse Cynthia Frankel also appear on the program and provide their
perspectives on the trial based on their experiences in a community practice
setting. The four patients we interviewed describe their reactions to the diagnosis
of breast cancer and why they chose to participate in a research study.
 |
 |
A few weeks later I thought of these patients while sitting with the multitudes at ASCO in Orlando on May 16, staring wide-eyed as Kathy Miller, Eric Winer, Edward Romond, Edith Perez, Martine Piccart- Gebhart and George Sledge described and discussed the very impressive benefits of treatment with bevacizumab/ paclitaxel in the first-line metastatic setting (ECOG-E2100) and the astonishing (as per George) benefits of trastuzumab with chemotherapy in the adjuvant setting (NSABP-B-31, NCCTG-N9831 and HERA).
The only one of these presenters who has not appeared on this series is Edward Romond, who sat down with me immediately after the ASCO session to review the historic data he presented on the combined NSABP-NCCTG adjuvant trastuzumab analysis. It turns out that Ed is a faithful listener of Breast Cancer Update as he winds through the roads of Kentucky. |
 |
The most memorable moment from Dr Romond’s interview was his recounting
of a patient who entered the trial several years ago. This mother of an 11-year-old
son had 25 positive axillary nodes and wished to enter NSABP-B-31 but was
concerned that her automobile would not be able to make the weekly four-hour
round-trip sojourn to Lexington to receive therapy. Both Ed and the patient
anguished about the potential lost opportunity to advance oncologic science and
possibly reduce the patient’s high risk of recurrence.
Dr Romond asked the young woman if someone in her family might drive her
back and forth to the clinic, and this prompted the patient to contact her father in
Texas, who immediately purchased a car to allow his daughter to enter the study.
The patient was randomly assigned to the chemo-trastuzumab arm of B-31 and
tolerated therapy without difficulty. Three years later, she remains free of recurrence,
and based on the data Ed presented at Orlando, it seems quite likely that
this may be a direct outcome of the administration of trastuzumab.
Ed’s compelling interview will be on the next issue of our series along with a
very memorable chat with George Sledge, who moderated the historic ASCO
session and delivered the penultimate presentation — a discussion of the clinical
and research implications of the data on adjuvant trastuzumab. I’ve had the
honor of interviewing George a number of times over the years, and when I
shot him an email a few days before ASCO requesting that he again meet with
me, I figured he would be too busy being probed by the Today Show and other
hungry media mouths to have enough time for the usual 75 minutes we allocate
for interviews.
Sure enough, George’s schedule was totally booked, but taking a very deep
breath, I modestly suggested that we meet at 6:00 AM on May 17, the day after
the ASCO “education” session. His short but acquiescent email reply made my
day: “Cruel and unusual punishment…but OK.”
When we sat down to chat, it seemed like the dawn of a new day in many ways. I
mentioned to George that the conversation reminded me a bit of an interview in
December 2001 with Mike Baum, just moments after his first presentation of the
ATAC trial data. It is interesting to imagine that if tamoxifen and other hormonal
therapies never existed, and the ATAC trial were the first formal randomized
test of endocrine therapy versus control (like the trastuzumab trials),
the magnitude of benefit would have been very similar to what was observed
with trastuzumab.
During the May 16 ASCO “education session,” every trial result presenter
utilized a closing slide acknowledging all who contributed. Topping each list
was a “thank you” to patient participants.
Damn right. Patients understand better than anyone the cruel impact of this
disease, and it gives them some comfort to be part of the problem’s solution. Sandra’s four patients typify the heterogeneity of people who place their bodies
on the front lines of this war.
The first patient I met is a frail septuagenarian from Chicago whose decision last
summer to retire to the tropical paradise of South Florida seemed questionable
when she and her husband were greeted by four consecutive hurricanes and an
abnormal mammogram, which led to a diagnosis of DCIS.
“We’ve been married 52 years,” she said with a proud smile, “but with all the
chaos, I thought our marriage was going to end. But it turned out that our
relationship was enhanced a great deal. My husband was with me through
everything, and when I didn’t feel well during radiation therapy and didn’t want
to take the pills for the study, he said, ‘You have to take those pills! Come what
may, you have to take those pills.’ So he’s been right at my side, and we actually
have become much closer.”
This spunky lady chose to enter B-35 because “there is just not enough that
people can do to stop the scourge of breast cancer. I want to protect my kids and
my grandchildren, and I’m very, very adamant about that.” This view is echoed
by the other three patients on the DVD.
Two of the women are in their early fifties and participate in NSABP-B-38,
comparing adjuvant therapy with TAC versus dose-dense AC T versus dosedense
AC T versus dosedense
AC T/gemcitabine. Both women had recently completed dose-dense
AC T/gemcitabine. Both women had recently completed dose-dense
AC T/gem and looked fairly well, which bodes favorably for the tolerance of
the experimental arm of this important study. T/gem and looked fairly well, which bodes favorably for the tolerance of
the experimental arm of this important study.
These patients became tearful when asked why they chose to enter the trial,
and it was clear that the basis for this decision was doctor-patient trust. They
described an instant sense of warmth and comfort during their first meeting
with Dr Franco, and it is clear that research participation is an important source
of empowerment for these patients and their physician.
The fourth patient is a miracle. Several months ago, this 45-year-old woman
was shocked to discover that despite a lifetime of meticulous health consciousness,
she had been diagnosed with HER2-positive breast cancer with 11 positive
lymph nodes. Fully aware of the approximately four percent risk of cardiac
toxicity with adjuvant trastuzumab, she chose to enter NSABP-B-31 and was
randomly assigned to receive AC T/trastuzumab. T/trastuzumab.
Several days before the interview, during this woman’s third cycle of AC, Cynthia
Frankel phoned and asked the patient if she had seen or read the news during
the past couple of days. The patient had been busy with her family and job and
had not yet learned what Cynthia was about to tell her: B-31 had been closed
due to an unexpectedly dramatic beneficial effect observed in the trastuzumab-containing
arm.
This kind and sincere mother of two is now continuing treatment knowing that
trastuzumab will further reduce her risk of relapse by about 50 percent. (Peter
Ravdin must be chained to his computer right now trying to pound out new
numbers for Adjuvant! based on this revolutionary data set.)
My job is to be a skeptic and to ask research leaders probing, prosecuting attorney-like
questions, and while I complain a great deal about the lack of progress in
our field, May 16 was a mighty good day, and while we owe a lot to people like
Richard Margolese, Bernie Fisher and Norm Wolmark, these leaders will be the
first to tell you that it’s the docs and nurses in the trenches, like Sandra Franco
and Cynthia Frankel, and patients like their four courageous trial participants,
who may eliminate this cruel disease in the near future.
— Neil Love, MD
NLove@ResearchToPractice.net
Select publications
Baum M et al; ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen
versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast
cancer: First results of the ATAC randomised trial. Lancet 2002;359(9324):2131-9. Abstract
Baum M, on behalf of the ATAC Trialists’ Group. The ATAC (Arimidex, Tamoxifen, Alone or in
Combination) adjuvant breast cancer trial in postmenopausal (PM) women. San Antonio Breast
Cancer Symposium 2001;8. No abstract available
Cuzick J. Aromatase inhibitors in prevention — Data from the ATAC (arimidex, tamoxifen
alone or in combination) trial and the design of IBIS-II (the second International Breast
Cancer Intervention Study). Recent Results Cancer Res 2003;163:96-103. Abstract
De Laurentiis M et al. Targeting HER2 as a therapeutic strategy for breast cancer: A paradigmatic
shift of drug development in oncology. Ann Oncol 2005;16(Suppl 4):iv7-iv13. Abstract
Frisby KA et al. Clinical trial accrual patterns and barriers among newly diagnosed adult
patients at a community cancer center: A prospective study. Proc ASCO 2005;Abstract 6017.
Guarino MJ et al. Barriers exist to patient participation in clinical trials. Proc ASCO 2005;Abstract 6015.
Miller KD et al. E2100: A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab
as first-line therapy for locally recurrent or metastatic breast cancer. Presentation.
Proc ASCO 2005a. No abstract available
Perez EA et al. HER2 testing by local, central, and reference laboratories in the NCCTG N9831
Intergroup Adjuvant Trial. Proc ASCO 2004;Abstract 567.
Perez EA et al. NCCTG N9831: May 2005 update. Presentation. ASCO 2005. No abstract
available
Piccart-Gebhart MJ. First results of the HERA trial. Presentation. ASCO 2005. No abstract
available
Romond EH et al. Doxorubicin and cyclophosphamide followed by paclitaxel with or without
trastuzumab as adjuvant therapy for patients with HER-2 positive operable breast cancer — Combined analysis of NSABP-B31/NCCTG-N9831. Presentation. ASCO 2005. No abstract
available
Umutyan P et al. Overcoming barriers to accrual: An assessment of 1,187 cancer patients’ (Pts)
and caregivers’ awareness of and willingness to participate in cancer clinical trials (CCTs). Proc ASCO 2005;Abstract 6016.
Vogel CL, Franco SX. Clinical experience with trastuzumab (herceptin). Breast J 2003;9(6):
452-62. Abstract
Vogel VG et al. National surgical adjuvant breast and bowel project update: Prevention trials
and endocrine therapy of ductal carcinoma in situ. Clin Cancer Res 2003;9(1 Pt 2):495-501.
Abstract
|

