You are here: Home: BCU 5 | 2005: Power Point Journal Club
| |
|
|
| |

This PowerPoint Journal reviews recently published clinical research articles and presentations. In this issue, we review an article by Davide Mauri et al on a meta-analysis of neoadjuvant versus adjuvant systemic therapy and a report by Ivo Olivotto et al on a population-based validation of the Adjuvant! program for early breast cancer.
PowerPoint Journal Club slides are provided in two formats, in this monograph and on the enhanced CD. The slide presentation on the CD is designed for optimal viewing on a large screen in a dark room (below, right) and represents top-line data and information from the figures in this book. This format of PowerPoint can be difficult to read in print, and consequently, the version below has been designed to facilitate ease of reading and comprehension.

| 5.1 |
|
| |
|
 SLIDE 5.1 Interest in preoperative systemic therapy has increased because of its association with local tumor regression and reduction in the extent of local surgery required. This meta-analysis assesses potential advantages of neoadjuvant versus adjuvant systemic therapy in breast cancer treatment. SLIDE 5.1 Interest in preoperative systemic therapy has increased because of its association with local tumor regression and reduction in the extent of local surgery required. This meta-analysis assesses potential advantages of neoadjuvant versus adjuvant systemic therapy in breast cancer treatment.
|
| |
|
|
| 5.2 |
|
| |
|
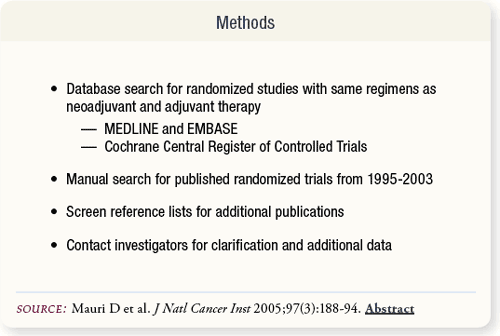

SLIDE 5.2 Mauri and colleagues identified suitable randomized studies for the meta-analysis by searching several web-based databases and oncology journals for publications of trials. The studies compared neoadjuvant with adjuvant therapy regardless of additional surgery or radiation treatment.
|
| |
|
|
| 5.3 |
|
| |
|
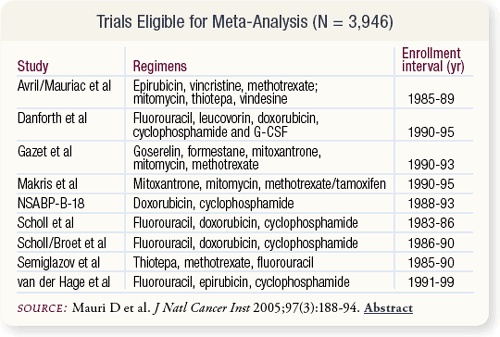

SLIDE 5.3 Nine trials were included, with outcomes of 1,933 and 1,928 patients randomly assigned to neoadjuvant and adjuvant study arms, respectively. Cancer stage, tumor size and nodal status varied across studies. Pre- and postmenopausal patients were included in all but one trial with only premenopausal patients.
|
| |
|
|
| 5.4 |
|
| |
|
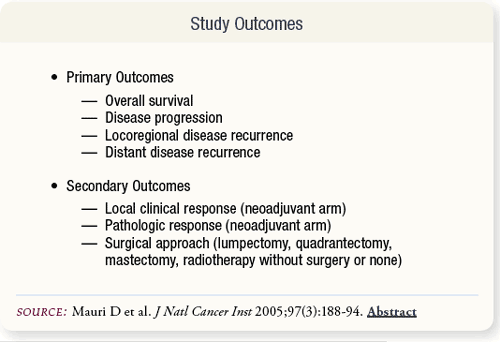

SLIDE 5.4 The analysis included the primary outcomes of death from any cause, disease progression, locoregional disease recurrence and metastases. Secondary outcomes included local clinical response, pathologic response in the neoadjuvant arm and the surgical approach in each study arm.
|
| |
|
|
| 5.5 |
|
| |
|
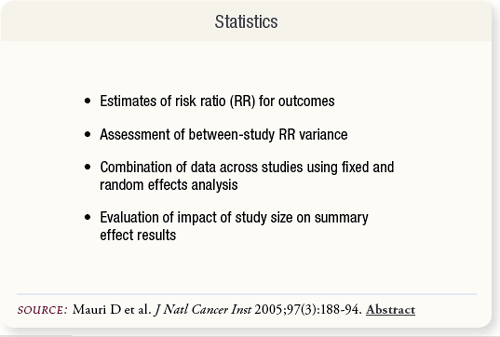

SLIDE 5.5 The meta-analysis was conducted using risk ratio (RR) estimates. An assessment of the variance across studies was made to determine heterogeneity between RRs, and data were combined using fixed and random effects methods. Trial size effects on results were also examined.
|
| |
|
|
| 5.6 |
|
| |
|
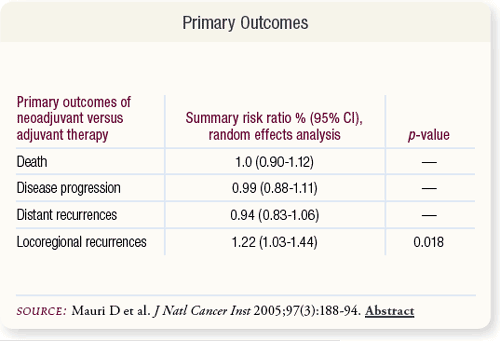

SLIDE 5.6 Primary outcomes between the two study arms were the same except for a 22 percent significant increase in relative risk of locoregional recurrences associated with neoadjuvant treatment.
|
| |
|
|
| 5.7 |
|
| |
|
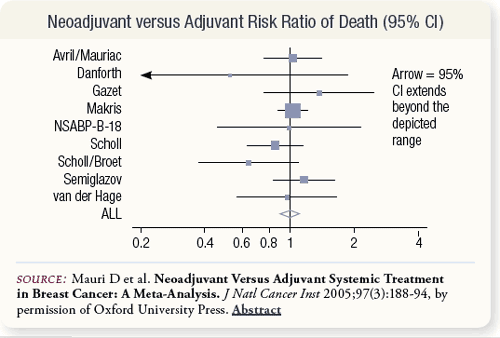

SLIDE 5.7 There was no difference in death rates between the neoadjuvant and adjuvant treatment arms. The summary risk ratio was 1.0 with a 95 percent confidence interval of 0.90 to 1.12.
|
| |
|
|
| 5.8 |
|
| |
|
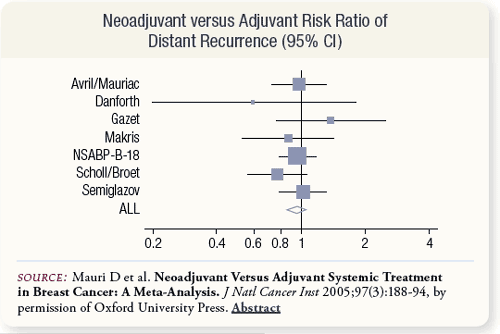

SLIDE 5.8 As with the data on mortality, no difference in distant recurrence rates between neoadjuvant and adjuvant treatment arms was observed across trials. The summary risk ratio was 0.94 with a 95 percent confidence interval of 0.83 to 1.06.
|
| |
|
|
| 5.9 |
|
| |
|
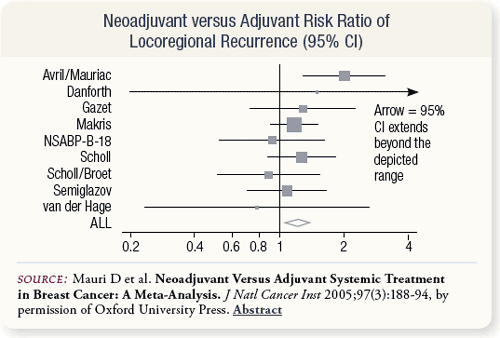
SLIDE 5.9 Locoregional recurrence was the only primary outcome associated with a statistically significant difference in rates between treatment arms across studies. The summary risk ratio was 1.22 (p = 0.015), indicating a 22 percent increased relative risk for locoregional recurrence with neoadjuvant treatment. |
| |
|
|
| 5.10 |
|
| |
|
SLIDE 5.10 Summary estimates were not made for any of the secondary outcome measures because of a high degree of variance in rates across studies.
|
| |
|
|
| 5.11 |
|
| |
|
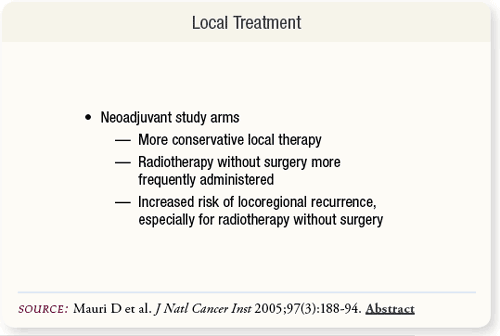
SLIDE 5.11 Neoadjuvant arms had higher rates of conservative local therapy. Radiotherapy without surgery was administered more frequently in neoadjuvant arms. Increased risk of locoregional recurrence was associated with neoadjuvant treatment, especially when radiotherapy was administered without surgery.
|
| |
|
|
| 5.12 |
|
| |
|
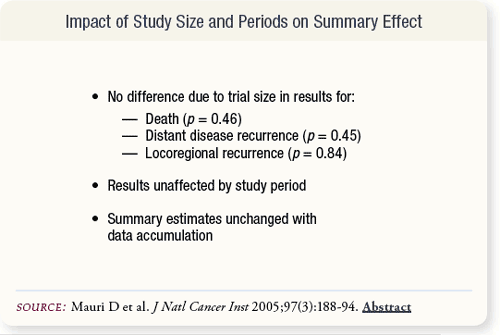
SLIDE 5.12 Study size did not affect outcome results for survival, distant disease or locoregional recurrence, although disease progression appeared to be more favorably associated with neoadjuvant treatment in smaller studies. Results also did not change with the addition of data over time.
|
| |
|
|
| 5.13 |
|
| |
|
SLIDE 5.13 These results cannot be extrapolated to agents of greater potency or different modes of action that have not been evaluated in trials comparing neoadjuvant to adjuvant therapy.
|
| |
|
|
Select publications
| 6.1 |
|
| |
|

SLIDE 6.1 Adjuvant! is a tool developed to objectively predict the absolute benefit of adjuvant systemic therapy for early breast cancer. This report assesses the validity of Adjuvant! based on a comparison of predicted estimates with observed outcomes for a population of women with Stage I or II breast cancer.
|
| |
|
|
| 6.2 |
|
| |
|
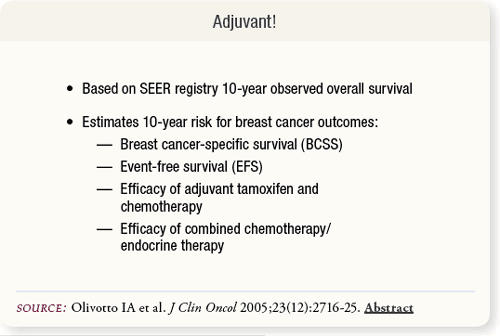
SLIDE 6.2 Adjuvant! estimates 10-year risks for breast cancer outcomes of BCSS, EFS and efficacy of adjuvant tamoxifen, chemotherapy and combined chemotherapy/endocrine therapy. It is based on Surveillance, Epidemiology and End Results (SEER) registry data of breast cancer diagnoses between 1988 and 1992.
|
| |
|
|
| 6.3 |
|
| |
|
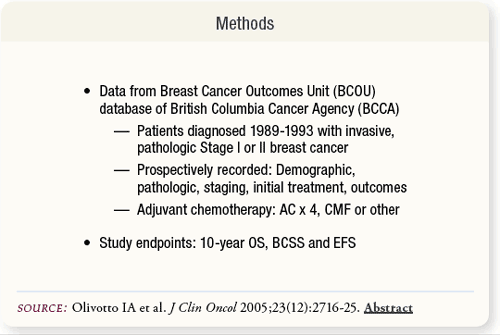
SLIDE 6.3 Seventy-five percent of patients from British Columbia with newly diagnosed breast cancer were referred to the BCCA between 1989 and 1993. From their database, data were gathered for patients’ demographic, pathologic, staging, initial treatment and outcome information for this comparison study.
|
| |
|
|
| 6.4 |
|
| |
|
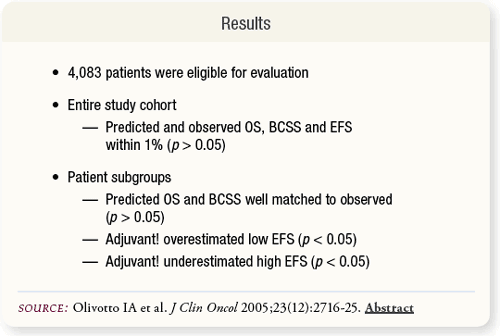
SLIDE 6.4 Predicted outcomes for OS, BCSS and EFS were determined with Adjuvant!. Results were compared with observed values. LVI presence was an important prognostic factor in the BCOU data but was not automatic in the Adjuvant! algorithm. Its inclusion required the PFIC feature. |
| |
|
|
| 6.5 |
|
| |
|
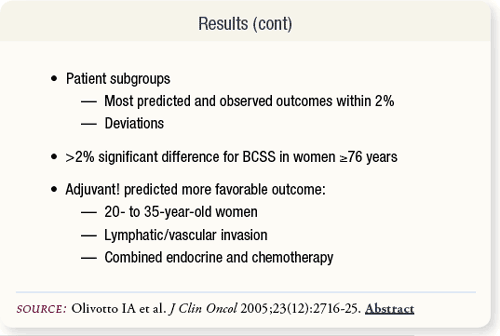
SLIDE 6.5 Overall predicted and observed outcomes were within one percent for OS, BCSS and EFS. With patient subgroups, the predicted and observed OS and BCSS were not different. However, Adjuvant! overestimated low EFS and underestimated high EFS.
|
| |
|
|
| 6.6 |
|
| |
|

SLIDE 6.6 Adjuvant! also overestimated BCSS, OS and EFS in women younger than age 35 years or with lymphatic or vascular invasion or with combined endocrine and chemotherapy.
|
| |
|
|
| 6.7 |
|
| |
|
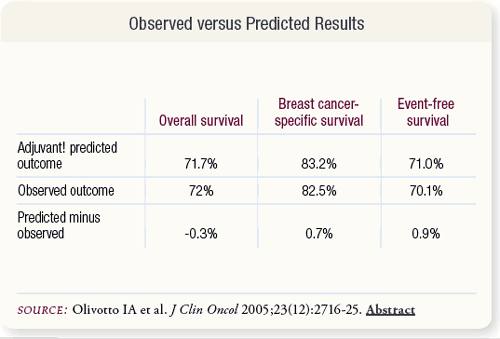
SLIDE 6.7 For the entire study cohort, the average predicted and observed outcomes for 10-year OS, BCSS and EFS were within one percent and were not significantly different (p > 0.05).
|
| |
|
|
| 6.8 |
|
| |
|
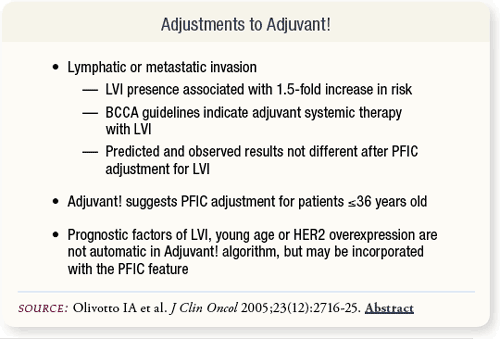
SLIDE 6.8 BCCA required treatment for patients with LVI, so they were included in the subgroup of patients receiving adjuvant systemic therapy. Use of Adjuvant!’s PFIC to adjust for this increased risk factor resulted in insignificant differences between outcomes. Similar adjustments are recommended for young age.
|
| |
|
|
| 6.9 |
|
| |
|
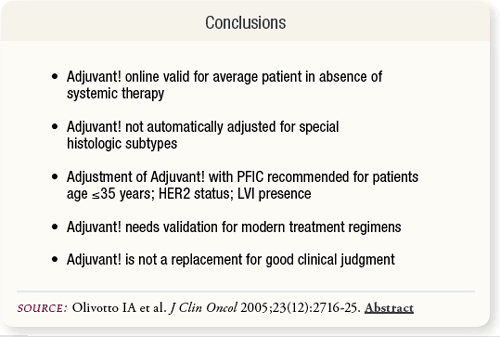
SLIDE 6.9 Adjuvant! performed reliably. To derive reliable predictions of prognosis without adjuvant systemic therapy and absolute benefits of adjuvant systemic therapy, patients younger than 35 years or with additional adverse prognostic factors require risk adjustments using the PFIC feature.
|
| |
|
|
| 6.10 |
|
| |
|
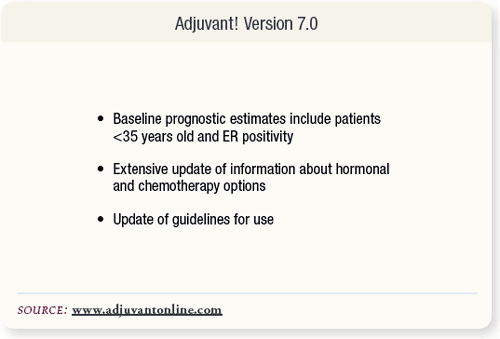
SLIDE 6.10 Since the acceptance of Olivotto’s article for publication, the Adjuvant! program has been updated. Version 7.0 has added the prognostic factors of young patients < 35 years and ER positivity to the baseline prognostic estimates. Updated information about hormonal and chemotherapy options was also added.
|
| |
|
|
Select publications
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

