J Michael Dixon, MD |
EDITED COMMENTS |
 Response to neoadjuvant systemic therapy Response to neoadjuvant systemic therapy
The number of patients receiving neoadjuvant endocrine therapy has increased significantly, and many oncologists who’ve tried this approach and found that it worked have adopted this strategy. I believe more physicians should be utilizing this because it’s effective at downstaging some large tumors, making inoperable tumors operable.
When we’re selective and treat only patients with ER-rich tumors, meaning Allred scores 6, 7 and 8, the number of patients who progress or actually fail to respond is very small. We treated approximately 250 such patients with neoadjuvant endocrine therapy, and only three or four of the patients had disease progression.
We have also learned that we can treat patients longer than three or four months with neoadjuvant therapy and see continued response. We’ve treated patients for up to a year and found that the number of patients with a complete response continues to rise the longer we treat them. If the tumor is shrinking but still not small enough for breast-conserving surgery at three or four months, continuing therapy will give added benefit, and eventually, most of these tumors will become small enough for breast conservation.
Neoadjuvant endocrine therapy versus chemotherapy
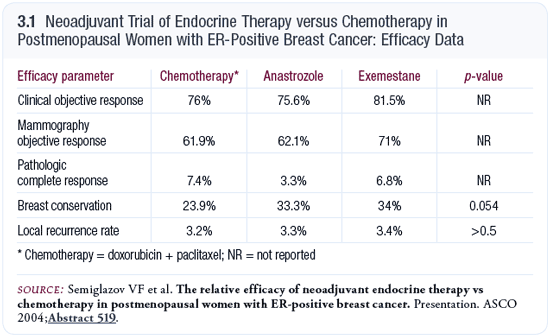
At ASCO in 2004, Semiglazov presented data from a small neoadjuvant study in which approximately 120 older postmenopausal women with ER-positive breast cancer were randomly assigned to receive doxorubicin/paclitaxel or an aromatase inhibitor — either anastrozole or exemestane (Semiglazov 2004). The response rates were in the 80 percent range and statistically similar whether the patients received endocrine therapy or chemotherapy (3.1).
Interestingly, the study revealed that the rate of breast-conserving surgery was higher in women who had received endocrine therapy. It was a small number, so it didn’t quite reach statistical significance, but the p-value was 0.054. I believe the reason for this is related to the way the tumor responds to neoadjuvant therapy. At ASCO 2005, we presented data showing that we’re significantly more likely to be successful performing breast-conserving surgery after neoadjuvant endocrine therapy than chemotherapy. One reason for this is that approximately 20 to 30 percent of patients who respond well to neoadjuvant chemotherapy are left with multiple islands of tumor scattered throughout an area of the breast that corresponds to the size of the original tumor, whereas the pattern following neoadjuvant endocrine therapy is that the tumor shrinks and implodes.
Tolerability of neoadjuvant systemic therapy
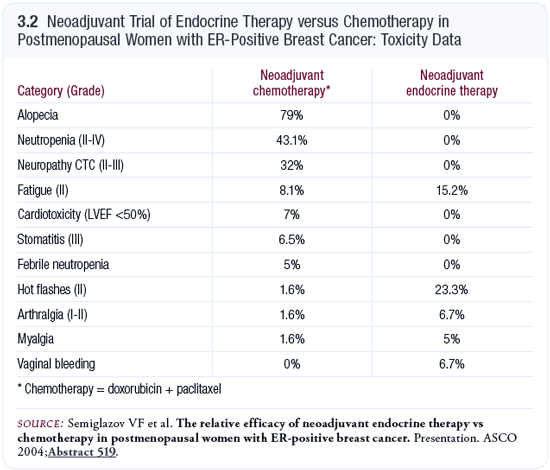
One interesting aspect of the Semiglazov series was the side-effect profiles (3.2). In the patients randomly assigned to chemotherapy, many experienced neutropenia, some developed febrile neutropenia, a large percentage lost their hair and a significant number experienced neuropathy. On the other hand, the toxicities from endocrine therapy consisted of hot flashes and muscular aches and pains. Our impression is that elderly patients tolerate endocrine therapy much better than chemotherapy.
This study did not reveal any toxicity differences between anastrozole and exemestane. We’re conducting a number of randomized studies comparing anastrozole, letrozole and exemestane, and it’s fairly clear that the side-effect profiles are different. With letrozole, the biggest side effect is fatigue; with anastrozole, we see more muscular aches and pains and some nausea. With exemestane, patients experience more diarrhea.
Neoadjuvant systemic therapy to reduce spread of cancer secondary to surgery
We presented a study at San Antonio in which over 200 patients were randomly assigned to receive either anastrozole or letrozole for 14 days prior to surgery (Murray 2004). We were examining biological factors in the tumor and found that proliferation was reduced between 80 and 84 percent in absolute terms within those 14 days.
We saw that within a few days of starting an aromatase inhibitor, we can switch off proliferation, so our strategy now in postmenopausal patients with invasive, ER-positive breast cancer is to begin an aromatase inhibitor straight away. If one is concerned that surgery spreads cancer, then it’s my view that if cancer cells are dying as a result of this approach, they are much less likely to take hold and metastasize.
A study reported to the Association of Surgeons in the United Kingdom examined patients who’d been given preoperative tamoxifen and found that they did better in terms of recurrence than patients who were started on routine adjuvant tamoxifen after surgery. It wasn’t a large study, nor was it randomized, but it’s anecdotal evidence, and scientifically it makes sense.
Treating patients with aromatase inhibitors doesn’t increase their risk of deep vein thrombosis or pulmonary embolus, so they can be given safely before surgery. It also allows patients to choose when to have an operation. Since they are on treatment, they can go on holiday with no rush to undergo surgery. Also, when the patient leaves the office, they already have a treatment, and you can tell them that by tomorrow their tumor will have started to shrink. Patients like that approach, and psychologically, we have found it to be a tremendous benefit.
Efficacy data from ATAC and BIG FEMTA/BIG 1-98
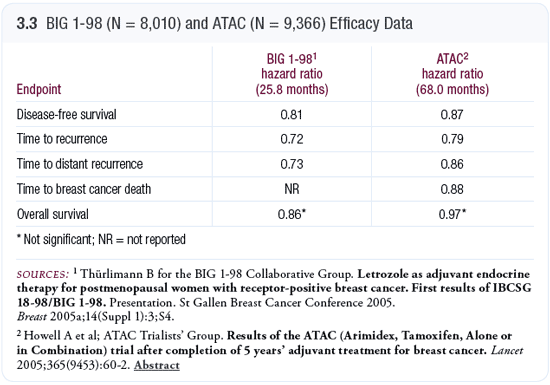
The data from the ATAC and BIG 1-98 trials are difficult to compare for a number of reasons (ATAC Trialists’ Group 2005; Thürlimann 2005a, b; [3.3]). The percentage of patients with positive nodes was 34 percent in the ATAC trial versus 41.3 percent in the IBCSG-1-98 trial. The percentage of patients who received chemotherapy was 21.3 percent in ATAC versus 25.3 percent in IBCSG-1-98. I believe that might be important because the overview suggested patients benefit more from hormonal therapy given alone than when combined with chemotherapy.
Secondly, the BIG 1-98 data are a short-term analysis — follow-up is only 25.8 months, whereas for the ATAC trial the follow-up is five years — and some concerns exist as to how the BIG 1-98 data are being analyzed. The trial has four arms, and patients who switched therapy after two years were included in the analysis, but only up until the time when they were switched. That’s a bit unusual, because one would expect some of the benefit from the first two years of tamoxifen and letrozole to continue.
BIG 1-98 did show quite clearly that it is more beneficial to use an aromatase inhibitor than tamoxifen when treating patients early, and it suggests that at least some patients — possibly a large percentage of patients — should receive aromatase inhibitors up front. If we don’t give patients aromatase inhibitors initially, then a large number will recur in the first two years. This was seen in the ATAC trial also.
Cardiac toxicity and safety data from the ATAC and BIG 1-98 trials
BIG 1-98 revealed a high incidence of hypercholesterolemia with letrozole, and the number of cardiac deaths was doubled in the patients receiving letrozole (Thürlimann 2005a, b). The numbers were small — 26 deaths on letrozole and 13 on tamoxifen — and it’s important to remember that despite the cardiac deaths, more patients were alive on letrozole than on tamoxifen, so it was beneficial in terms of overall breast cancer mortality. We saw similar findings of excessive cardiac events with exemestane.
Later this year, the ATAC data on adverse events in terms of heart effects and the number of patients reported to develop hypercholesterolemia will be presented. My understanding is that no major adverse effects are being seen with anastrozole. I’ve always believed letrozole is more potent, and we may not want the most potent drug in the adjuvant setting, because the most potent drug may have more adverse events. The letrozole data concerns me a bit. Clearly, we need longer-term data before we start using letrozole up front for five years. The data that have been released from ATAC in terms of cardiac deaths do not suggest excessive deaths with anastrozole, and the overall hazard ratio for breast cancer deaths in ATAC is favorable, but the overall mortality is 0.97 (ATAC Trialists’ Group 2005).
Another interesting aspect of the BIG 1-98 and ATAC findings was that the annual fracture rates were identical. We are currently conducting an open-label study examining letrozole, exemestane and anastrozole and their effects on lipids, clotting and bone. The results will be quite important. However, based on the current data, I believe most postmenopausal patients with ER-positive breast cancer should receive anastrozole front line for adjuvant therapy.
Endocrine switching trials in the adjuvant setting
The combined analysis of the Austrian (ABCSG Trial 8) and the German (ARNO 95) trials, in which patients were switched to anastrozole after two years of adjuvant tamoxifen, is difficult to compare to the Intergroup Exemestane Study (IES), in which patients were switched to exemestane (Jakesz 2004, Coombes 2004).
In the IES, 44.2 percent of women had node-positive disease and 32.7 percent received chemotherapy, whereas in the combined analysis, 25.9 percent of patients had positive nodes, none of them received chemotherapy and the majority of patients had Grade I or II disease. Perhaps, then, it shouldn’t be surprising that a marked benefit was seen with anastrozole, with a hazard rate for breast cancer events of 0.60 and a mortality hazard ratio of 0.76, almost reaching statistical significance. However, in the United States, I believe many of these patients would have received chemotherapy and, therefore, we would have seen slightly less benefit from switching therapies.
I believe postmenopausal patients with ER-positive tumors who have been on tamoxifen for a couple of years will generally benefit from switching to either anastrozole or exemestane. Based on the data, I believe both are effective.
As for toxicities, in the IES data we did see more myocardial infarcts with exemestane than with tamoxifen, which corresponds to what we have seen with letrozole. Tamoxifen is protective against myocardial infarcts, so we might just be seeing the tamoxifen preventative effect. As for the IES bone subprotocol, they didn’t find exemestane to be any better than the other aromatase inhibitors in terms of protecting bone (Coleman 2004).
Trials of fulvestrant in premenopausal women
In premenopausal women, we’re doing some interesting work with fulvestrant. Part of the problem with fulvestrant is that the doses used in postmenopausal women did not show a benefit in premenopausal women. In our study, which we will probably present in San Antonio later this year, we’re comparing preoperative tamoxifen to preoperative fulvestrant at a dose of 750 mg, which is three times the dose currently used in postmenopausal women. From our preliminary data, it is clear that fulvestrant is having some effect on these tumors. The hope is we might have another agent that is useful in premenopausal women.
Select publications
 |
Dr Dixon is a Consultant Surgeon and Senior Lecturer with the Academic Office of the Edinburgh Breast Unit at Western General Hospital in Edinburgh, United Kingdom. |
|

