Nancy E Davidson, MD |
EDITED COMMENTS |
 Regulation of estrogen receptor expression Regulation of estrogen receptor expression
I’m very interested in hormone-responsive breast cancer and struck by the fact that clinically, approximately 25 percent of breast cancers lack the estrogen receptor. We have been thinking about why that is and are exploring mechanisms for regulation of the estrogen receptor.
One of the mechanisms that we’re interested in is the concept that epigenetic silencing mechanisms might lead to loss of estrogen receptor expression. These changes modify expression of a gene and are potentially reversible, unlike mutations, which are permanent, and we are thinking about whether we can take advantage of this therapeutically.
In addition, a lot of genes are epigenetically silenced in cancer in general, not just breast cancer, and these changes are felt to be a hallmark for cancer. We can detect these changes molecularly on small specimens, and so the possibility exists that they might be useful as risk assessment or screening tools.
It’s believed that most normal mammary epithelial cells actually have very little estrogen receptor expression. The Baylor group has suggested that some premalignant lesions, such as atypical hyperplasias, are rich in estrogen receptor expression, and we know that many ductal carcinoma in situ lesions, because we measure it now, are estrogen receptor-positive. However, we don’t know how this progression takes place and whether it represents two kinds of invasive breast cancer that deviate early or whether one logically progresses into the other.
Histone deacetylase inhibitors to modify gene expression
In breast cancer, the estrogen receptor is generally intact. The problem in estrogen receptor-negative breast cancer is that the tumor doesn’t transcribe the RNA, so it doesn’t make the protein. We were very interested in these epigenetic modifications — histone modifications or DNA methylation — as a way of silencing estrogen receptor gene expression, and using PCR-based techniques, it appears this happens in many cancers.
It’s interesting that this exists and that in the laboratory we can reverse it. We can use histone deacetylase inhibitors — like suberoylanilide hydroxamic acid (SAHA) and a number of compounds that are being tested in Phase I and Phase II trials — and we can also use the DNA methyltransferase inhibitor azacitidine, which has just been FDA approved for myelodysplastic syndromes. We have a lot of preclinical cell culture data, so now we’re moving on to animal models.
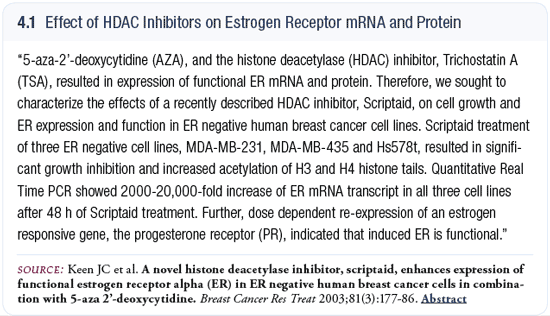
We are conducting our first clinical trial with preoperative SAHA in healthy women with primary breast cancer. A core biopsy will be taken initially for research purposes, and then patients will take three days of SAHA, which is an oral agent. At the time of the definitive surgery, post-treatment tissue will be examined for the usual endpoints — change in Ki67, change in histones — because we expect it will modify the histone acetylation. It’s largely an exploratory trial, the question being: What gene expression patterns and profiles and proteome patterns are modified by this histone deacetylase inhibitor? We have never done this before, and I hope to see changes in the estrogen receptor occur, because I believe the estrogen receptor is just one of many genes that are epigenetically modified (4.1). We are not going to restrict this trial to estrogen receptor-negative patients. We hope we’ll have some and that will be one of several candidate genes we’ll be evaluating.
While I am interested in the estrogen receptor, others have noted that retinoic acid receptor beta is also epigenetically silenced in many breast cancers. If in the lab we can change that — turn it back on — then we can treat with retinoids, and there’s been a lot of interest in using various retinoids for treatment of malignancies, including breast cancer.
One hypothesis is that the cancer turned these genes off for a reason; it needed to inactivate them in order to move along its carcinogenic progression — a tumor suppressor gene, for example — so it is hoped that re-expressing these will result in tumor inhibitory or suppressor effects.
Neoadjuvant trials targeting breast cancer prevention
In our preoperative trials, we are taking advantage of the concept of the contralateral breast as a prevention test model. We have a trial in which postmenopausal women who are not going to receive adjuvant chemotherapy receive neoadjuvant anastrozole. All the patients have initial core biopsies of the contralateral breast, which is presumably an at-risk but healthy breast, and the biopsies are repeated six months after they begin anastrozole.
A variety of correlative studies will be performed, including evaluation of lipids and breast density. We hope to see a decrease in breast density and determine the impact on lipids with anastrozole. We will use those tissues for exploratory studies, looking for a gene that might be modulated by anastrozole in the breast tissue itself. Then, perhaps, we can establish suitable endpoints that could be used to shorten future chemoprevention trials.
We are about to start a parallel trial evaluating a statin. The interest in whether statins can reduce breast cancer risk basically comes from observations made in the cholesterol-lowering trials, where in some cases it appeared women who took these agents had a lower breast cancer risk. For that particular trial, we’re going to target women who have ER-negative breast cancer, because it would be too complicated to conduct in the context of ongoing hormone therapy.
Cardiovascular effects of aromatase inhibitors
Cardiovascular events are a potential issue with the aromatase inhibitors. However, we’re also less certain now about what impact estrogen itself has on the heart. We used to believe it was good for the heart and taking it away was bad, but then the data from the Women’s Health Initiative (WHI) indicated estrogen is probably not good for the heart (Rossouw 2002; [4.2]). As a result of the WHI, we might think estrogen withdrawal would be better for the heart, but considering the aromatase inhibitor trial data, that may not be the case. It’s a complex issue.

Avoiding taxane-associated adverse events with nab paclitaxel
Nab paclitaxel is an interesting drug. I’ve been thinking about it recently for a young patient with metastatic breast cancer who had a very difficult reaction to docetaxel a few years ago. She received a number of hormone therapies, and now she’s hormone resistant; she has also received a number of chemotherapies, including vinorelbine and capecitabine.
When she was in a trial that involved an anthracycline and docetaxel, she had an acute hypotensive reaction. We tried it again, and she had the same reaction, so we stopped the docetaxel and continued the anthracycline. We haven’t tried a taxane since. Now nab paclitaxel is being considered in the hope that she could get the benefits of a taxane without the adverse reaction.
The toxicity profile of nab paclitaxel appears to be better than the other taxanes, and premedication is not needed, which is a big plus. The other advantage is that the administration time is shorter. The problem with the Phase III trial was that it compared an every three-week schedule for nab paclitaxel and paclitaxel, whereas I usually give paclitaxel on a weekly schedule (O’Shaughnessy 2003).
Phase II studies of weekly nab paclitaxel have been reported (O’Shaughnessy 2004), but we don’t know how weekly paclitaxel and nab paclitaxel compare head to head. Nab paclitaxel is an intriguing drug, and it’s good to have an alternative that simplifies administration and minimizes toxicity.
Sequence of single chemotherapy agents in metastatic disease and the role of capecitabine
My philosophy in treating older versus younger women with chemotherapy is basically the same, but sometimes the choices of the patients are different. Many times in metastatic disease, we use all of the available therapies, so what we’re really deciding on is the order — what to start with. Many patients make that decision based on their personal values.
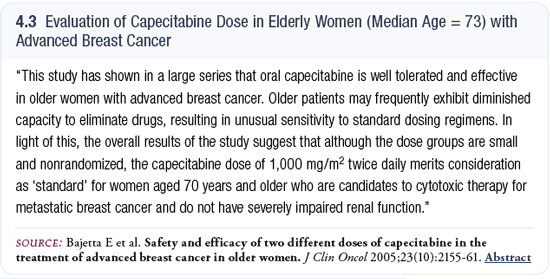
I find many of my older patients are attracted to capecitabine because it is an oral agent (4.3). Some of my younger patients think of intravenous therapy as more aggressive, and they prefer that strategy. But this perception is based on gut reaction rather than being reality based.
I am a big fan of capecitabine. Maybe it comes from being a “hormonal therapy person” who prefers pills to begin with, because I use capecitabine a lot for salvage chemotherapy in women who’ve already had an anthracycline and taxane for metastatic disease.
In oncology, we tend to remember our successes, but I have seen several very impressive responses with capecitabine in dire circumstances. I have had women on capecitabine for a considerable period of time with relatively good quality of life.
Select publications
 |
Dr Davidson is a Professor of Oncology, Breast Cancer Research Chair in Oncology and Director of the Breast Cancer Research Program at The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, Maryland. |
|

