|
You
are here: Home: BCU 3|2003: Nicholas
J Robert, MD

Edited comments by Dr Robert
US Oncology Phase III study of trastuzumab/paclitaxel
with or without carboplatin
The study in advanced breast cancer was spawned by the results
of the pivotal trial by Slamon and colleagues, in which the combination
of paclitaxel/trastuzumab improved the response rate to the 40
percent range and the time to progression to 6.9 months compared
to paclitaxel alone.
We couldn’t add doxorubicin to the paclitaxel/trastuzumab
combination because in the pivotal trial, 28 percent of patients
in the group given doxorubicin, cyclophosphamide and trastuzumab
had cardiotoxicity. We knew of preclinical synergy between the
taxanes and carboplatin, as well as three first-line therapy trials
showing response rates between 52 percent and 62 percent produced
by the combination of paclitaxel and carboplatin. Therefore, adding
carboplatin seemed an obvious next step in evaluating the paclitaxel/trastuzumab
combination.
Eligibility and protocol
We recruited 196 patients with Stage IV, HER2-positive breast
cancer, of whom 191 were eligible and 186 were evaluable for response.
As in the trial by Slamon and colleagues, we accepted IHC 2+ and
3+ patients, but as the data became available, we found that only
30 percent of the IHC 2+ patients were FISH positive. Therefore,
we changed our eligibility requirements so that patients who were
IHC 2+ also had to be FISH-positive. Patients had to have measurable
disease and a normal left ventricular ejection fraction. They were
ineligible if they received adjuvant taxanes or more than 360 mg/m2
of doxorubicin.
Patients were randomized to receive trastuzumab/paclitaxel, the
successful arm of the pivotal trial, or the combination plus carboplatin.
Paclitaxel was administered at 175 mg/m2 over three hours every
21 days, trastuzumab was administered at a standard loading dose
of 4 mg/kg followed by weekly 2 mg/kg, and carboplatin was administered
at an AUC of six every 21 days. As in the pivotal trial, physicians
had to give six cycles of chemotherapy, but could discontinue chemotherapy
and continue the trastuzumab after that.
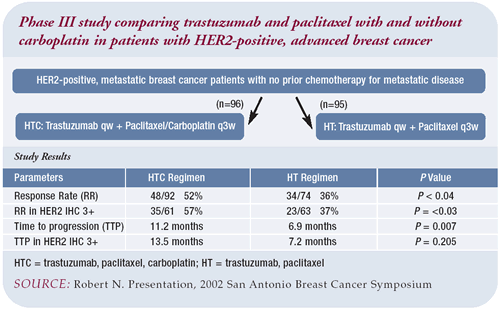
Trial results
The addition of carboplatin improved both the response rate and
time to progression. The primary endpoint was the response rate,
which improved from 36 percent with the two-drug regimen to 52
percent with the addition of carboplatin, with a P value of 0.04.
We stratified IHC 2+ and 3+ patients, and the response rate in
the 3+ patients jumped to 37 percent with the two-drug regimen
and to 57 percent with the addition of carboplatin, with a P value
of 0.03. FISH data was collected retrospectively and, although
the comparison is not powered for significance, we saw a similar
trend as in the IHC 3+ patients — response rates of 39 percent
and 59 percent with the two- and three-drug regimens, respectively.
Time to progression was a secondary endpoint in the trial. The
time to progression in the trastuzumab/paclitaxel control arm was
similar to what was seen in the pivotal trial by Slamon and colleagues.
The addition of carboplatin increased the time to progression from
6.9 months to 11.2 months. Looking only at the IHC 3+ patients,
we saw a similar improvement (7.2 months increased to 13.5 months);
similar results were seen in the FISHpositive patients as well.
We looked at survival, although it was early to do so as over
120 patients are still alive. The preliminary analysis shows a
trend for improvement with the three-drug regimen. In the IHC 3+
patients, we saw an improvement in survival, with a P value of
0.06, approaching 0.05, and the FISH-positive population showed
a similar trend. It will be important to see if the survival advantage
persists.
Tolerability and safety data
The trastuzumab/paclitaxel/carboplatin regimen was well tolerated.
The only significant difference in toxicity was increased myelosuppression,
which we expected to see from adding carboplatin. However, there
were no significant differences in terms of serious complications,
such as infectious complications, significant neutropenia or fever.
Other toxicities, such as neuropathy, allergic responses, nausea
and arthralgias, were comparable in both arms.
It is important to note that we did not use prophylactic growth
factors or attempt a dose-dense trial. We utilized dose reduction
or dose delay when needed. In responding patients, only about 25
percent continued treatment beyond six cycles, so, there are a
number of important caveats in administering this regimen in order
to get the benefits and avoid unacceptable toxicities.
Implications for research
One of the questions our trial evoked was: Could we achieve the
same results by treating patients with paclitaxel/trastuzumab and
switching to carboplatin and trastuzumab when they progress? Historically,
carboplatin is not a very effective agent when given outside the
first-line setting, with response rates in the range of 10 percent;
but, it’s possible that in combination with trastuzumab it’s
a different drug. A small study from UCLA using cisplatin in heavily
pretreated patients showed about a 24 percent response rate. This
may be a strategy to consider in future clinical trials.
Edith Perez and the North Central Cancer Treatment Group, in
anticipation of positive results from our study, are looking at
giving paclitaxel and carboplatin weekly versus every three weeks.
The Breast Cancer International Research Group, in BCIRG-007, is
comparing trastuzumab and docetaxel with or without carboplatin
in FISH-positive patients. They hope to recruit over 500 patients
and have about 70 to date. We won’t be able to compare the
different taxanes with these two studies, but we will be able to
evaluate the impact of carboplatin on the trastuzumab/taxane regimen.
We also know that the combination of trastuzumab and vinorelbine
has activity. Currently there’s a randomized Phase II trial
in Boston, comparing that combination to trastuzumab and a taxane.
It will be interesting to compare the efficacy and toxicity of
this two-drug regimen (trastuzumab/ vinorelbine) with the three-drug
regimen (docetaxel/trastuzumab/ carboplatin).

Next page
Page 1 of 2
|
|
