| You
are here: Home: BCU 3|2003: Nicholas
J Robert, MD

Trastuzumab as first-line therapy
for metastatic breast cancer
I am not aware of any evidence supporting sequencing a non-trastuzumab
combination followed by a trastuzumab combination in chemotherapy-naïve
patients with HER2-positive metastatic disease. Rather, the pivotal
trial comparing chemotherapy plus or minus trastuzumab showed improvement
in response rate, time to progression and survival when trastuzumab
was added. In addition, over 50 percent of the patients who did
not receive trastuzumab initially, received it subsequently, and
did not get the same survival benefit.
In patients with HER2-positive, ER-negative metastatic disease,
single-agent trastuzumab is a reasonable first step. Both Chuck
Vogel in the first-line setting, and Melody Cobleigh in second-
and third-line settings, have experience with single-agent trastuzumab
in patients with indolent ER-negative disease. In a trial of single-agent
trastuzumab, the Sarah Cannon Cancer Center investigators saw a
greater than 50 percent clinical benefit. Patients who crossed
over to adding carboplatin and paclitaxel exhibited an increased
response rate, but there is probably a subset of patients who could
be treated with trastuzumab alone for a while to see how they do.
Single-agent trastuzumab versus trastuzumab
with chemotherapy
The decision to use trastuzumab sequentially versus concomitantly
with chemotherapy is based on issues such as extent of metastatic
disease and the time between diagnosis and progression. In a younger,
relatively asymptomatic patient with bone metastases and a good
performance status, I don’t think there is compelling evidence
to use both chemotherapy and trastuzumab initially. There is no
randomized trial comparing sequential versus concomitant therapy
in such a patient, but in other settings comparing sequential versus
concomitant therapy with chemotherapy, concomitant therapy doesn’t
do any better in terms of survival.
Certainly there are patients with metastatic disease in whom
you feel chemotherapy is indicated, such as patients with significant
visceral or lifethreatening disease. Given the positive results
of the trials where trastuzumab was added to chemotherapy — improved
response rate, time to progression and survival — my approach
has been to give trastuzumab with the chemotherapy. Given our recent
Phase III trial results, I would use the carboplatin/paclitaxel
regimen.
BCIRG-006: Adjuvant trastuzumab trial
I am excited about the novel approach of BCIRG-006 for HER2-positive
patients in the adjuvant setting. In this trial, patients in the
control arm receive doxorubicin/cyclophosphamide followed by docetaxel.
The second arm is the same doxorubicin/cyclophosphamide regimen,
followed by docetaxel plus trastuzumab, continuing trastuzumab
weekly for one year. The third arm is quite innovative in that
it includes a taxane rather than an anthracycline. Patients receive
trastuzumab, docetaxel and carboplatin or cisplatin for six cycles,
followed by weekly trastuzumab for one year from the beginning
of therapy.
This trial has an accrual goal of 3,000 patients and over 1,000
patients are already enrolled. It requires patients to be FISH-positive,
which is probably the best predictor of interaction between trastuzumab
and chemotherapy. I think the results will be quite meaningful
to the future management of HER2-positive patients in the adjuvant
setting.
Implications of the ATAC trial results for clinical
practice
When I heard the ATAC trial data last year, I was impressed.
It’s a large trial of more than 9,000 patients, and the disease-free
survival benefit with anastrozole was credible. It was interesting
that the combination didn’t work, but anastrozole certainly
appeared superior to tamoxifen.
The results of the 47-month update show continued improvement
in diseasefree survival with actual improvement in the hazard rate
with time, which provides even more support for the use of anastrozole
in the adjuvant setting.
Currently, I uniformly recommend anastrozole to my patients at
high risk for recurrence. I also use anastrozole in patients who
are experiencing problems with tamoxifen — severe hot flashes,
weight gain or issues related to their uterine status. Occasionally,
I have had patients on anastrozole who switched to tamoxifen because
of arthralgias. Tamoxifen is still a reasonable choice in an older
patient with a low risk of recurrence.
I have not switched a patient who was doing well on tamoxifen
to anastrozole. I also have not used the other aromatase inhibitors
outside of a clinical trial, because the data is with anastrozole.
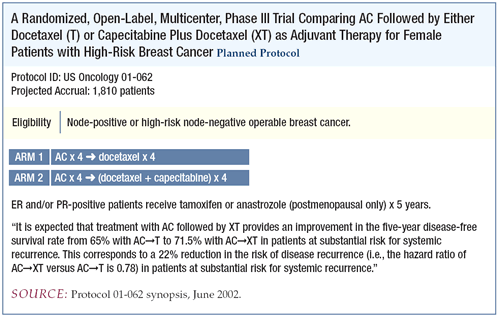
Adjuvant trial of capecitabine/docetaxel
Under the leadership of Joyce O’Shaughnessy, we are conducting
an adjuvant trial aimed at taking advantage of the biochemical
interaction between taxanes and capecitabine. Doxorubicin/cyclophosphamide
followed by docetaxel, the control arm, will be compared to the
capecitabine/docetaxel combination. This trial is based on the
US Oncology study that evaluated this combination in the metastatic
setting and showed improved outcome, including survival, in the
capecitabine/docetaxel arm.
The tolerability of the regimen is a real concern in the adjuvant
setting, so we lowered both the capecitabine and docetaxel doses
in the investigational arm of the adjuvant trial. Accrual has been
good to date and we have some early toxicity data.
CALGB-9741: Dose-dense versus conventional adjuvant
chemotherapy
The results of the dose-dense trial are exciting. We’ve
looked at dose for a long time and the strategies studied — increasing
cyclophosphamide from 600 mg/m2 to 2,400 mg/m2, increasing doxorubicin
from 60 mg/m2 to 90 mg/m2 and giving high-dose chemotherapy in
the adjuvant setting — were not successful.
However, dose density — giving the drugs every two weeks
versus every three weeks — has translated into a disease-free
survival advantage and, at this point, a survival advantage. I’m
waiting to see more data, but I will present the results from the
dose-dense trial to my patients, letting them know these are early
results and there is some tradeoff.
Treatment algorithm for patients with metastatic
breast cancer
Choosing a chemotherapy regimen for patients with metastatic
breast cancer depends on the pace of the disease. If someone with
indolent disease progresses on endocrine agents, switching them
to another oral agent such as capecitabine is a very attractive
option.
Capecitabine is generally well tolerated, especially now that
we have improved dosing. We now begin with 1,000 milligrams per
meter squared BID, rounding down as needed for 14 out of 21 or
28 days (two weeks on, one to two weeks off). In my experience,
this regimen is both well tolerated and efficacious.
Combination chemotherapy is a consideration in patients with
more aggressive disease. In a patient who’s had prior anthracyclines,
a taxane or taxane combination (such as taxane/carboplatin), especially
with the weekly schedule, is a well-tolerated and efficacious approach.
Use of fulvestrant in patients with ER-positive metastatic disease
I’ve used a fair amount of fulvestrant, and I find that it’s
well tolerated. Patients don’t have any problem coming in
once a month for their intramuscular injections. In terms of efficacy,
we’ve had patients experience stabilization of disease for
six months. What is nice about fulvestrant is that it offers another
option, especially for the patient who may be experiencing difficulty
tolerating their current endocrine therapy.
Targeting angiogenesis in the treatment of breast
cancer
Although it was disappointing that the response rates in the
bevacizumab trial did not translate into time to progression, I
would not get too discouraged. It reminds us that we need to know
the target in order to target therapy. Chemotherapy covers a number
of different tumor cells, and we don’t need to be as specific
with therapy. But, imagine if we had done the trastuzumab trials
without knowing to target HER2, IHC 2+ and 3+ patients — we
would not have seen the benefits of the therapy. Tamoxifen is another
good example of a targeted therapy; it works in ER-positive tumors,
but it took us a long time to figure that out.
In order to target angiogenesis, we need to identify tumors in
which angiogenesis is important, and we probably need to use a
multiagent approach to hit multiple targets. Our increased understanding
of the molecular biology of breast cancer has given us better insight,
and we need to step up to the challenge of developing targeted
strategies to take advantage of that biology.
EGFR inhibitors in the management of ER-negative
breast cancer
We know from the NSABP P-1 study that in the high-risk population,
tamoxifen reduces only ER-positive breast cancer incidence. Dr
Craig Allred, in analyzing NSABP B-24, found that patients with
ER-negative DCIS received no benefit from tamoxifen. What are the
strategies available to treat ER-negative breast cancer?
The role of EGFR inhibitors in the management of patients with
ER-negative breast cancer was discussed at the San Antonio meeting.
First, one has to be certain that the immunohistochemistry is properly
interpreted and the patient is truly ER-negative. Tamoxifen doesn’t
work in that group, but could an EGFR inhibitor — like gefitinib — potentially
work? Manipulating the EGF receptor tyrosine kinases is a potential
approach, but additional research needs to be done in this area.
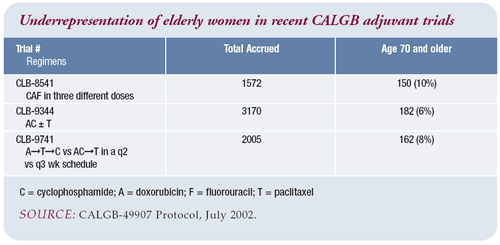
Participation of the elderly in clinical trials
I have a lot of respect for CALGB-49907 for both the trial design
and the idea of recruiting the elderly for clinical trials. It
will be interesting to see the results comparing capecitabine to
standard regimens. US Oncology also has a trial for the elderly,
comparing intravenous CMF versus weekly docetaxel.
When you look at previous trials, patients over 70 are underrepresented.
The accrual in both of these trials is slow, which may just be
the nature of treating an older population with other medical illnesses.
Maybe their ability to tolerate chemotherapy is less, although
there are studies that suggest that isn’t the case.
The number of eligible patients is probably smaller, and their
ability to come in for treatment may be a barrier. While both of
these trials are reasonable, the challenge is getting them done.
Development of a nonalopecia-causing breast cancer
regimen
The US Oncology breast committee is seeking to develop a regimen
that does not cause alopecia. While 20 years ago we had only a
handful of drugs to work with, we now have a number of drugs that
are not associated with alopecia — capecitabine, vinorelbine,
gemcitabine, doxorubicin HCL liposome injection — and a number
of combinations can be studied. It is worth noting that we don’t
do a good job of recording alopecia in clinical trials, and we
need better documentation.
It will take some time to find a successful nonalopecia-causing
regimen in the adjuvant setting — we certainly won’t
compromise survival to save hair. This is also important in the
metastatic setting. Breast cancer is a very private matter, and
alopecia makes it difficult to hide. Eliminating this side effect
of therapy would make breast cancer treatment easier for patients.
Select publications
Return
Page 2 of 2
|
