| You
are here: Home: BCU 3|2003: G
Thomas Budd, MD

Edited comments by Dr Budd
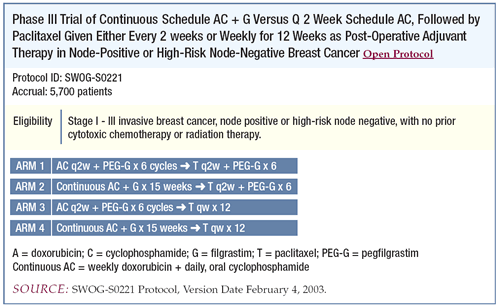
SWOG-S0221: A new Phase III adjuvant Intergroup
trial evaluating chemotherapy schedules
In SWOG-S0221, the combination dose-dense arm of CALGB-9741 was
selected for the initial randomization instead of the sequential
arm. Our rationale was to shorten the duration of treatment and
to make it more comparable to the AC regimen in the experimental
arm.
In the second randomization, we were originally going to compare
docetaxel alone to docetaxel plus capecitabine. There were a couple
of reasons we decided to compare paclitaxel every two weeks to
paclitaxel every week. First, the docetaxel/capecitabine combination
is being investigated in several other multicenter adjuvant trials.
Second, it was felt that we should preserve the control arm from
CALGB- 9741, which administered paclitaxel every two weeks. At
the end of SWOGS0221, we hope we will know the optimal way to administer
paclitaxel in the adjuvant setting.
SWOG-SO221: Rationale for daily oral cyclophosphamide
Daily oral cyclophosphamide seems to be superior. In the metastatic
setting, an EORTC trial comparing intravenous CMF to a regimen
with oral cyclophosphamide found the oral cyclophosphamide regimen — the
so-called “classical” regimen — to be superior.
Furthermore, in the adjuvant trials comparing CAF and CMF regimens,
only when cyclophosphamide is given in the same way — either
both arms receive intravenous or oral cyclophosphamide — is
there a difference seen with the addition of the anthracycline.
Why does the oral method of administration seem to be superior?
One reason could be dose density — you deliver more milligrams
in per unit time. By giving cyclophosphamide orally and doxorubicin
weekly, the dose density in terms of mg/m2 per week compared to
the accelerated regimen, at least for doxorubicin, is not necessarily
superior but is denser. Bob Livingston sometimes calls this the “dense
and denser” protocol, because we are giving what we believe
to be an effective dose of an anthracycline on a more frequent
schedule.
Metronomic chemotherapy
The third way of looking at this is the concept of metronomic
chemotherapy, which suggests that more frequent drug administration
at lower doses — sometimes even very low doses — might
actually be superior to higher doses given less frequently. It
appears that this advantage is based upon the antiangiogenic effects
of the chemotherapy. Giving a low dose of chemotherapy frequently
appears to optimize its antiangiogenic effects.
A number of preclinical models have evaluated this concept. When
cyclophosphamide-resistant cell lines are put into animals and
the animals are given cyclophosphamide on an infrequent bolus schedule,
responses are rarely seen. However, if cyclophosphamide is given
frequently (every six days) at a lower dose, responses may occur.
In these animal models, the results appear to be due to the antiangiogenic
effects. This metronomic schedule seems to be optimal for the addition
of other antiangiogenic factors.
Some clinical studies also support the concept of metronomic
chemotherapy. If you look back at the old Cooper regimen (CMFVP),
maybe that was the secret to its efficacy. In Europe, there was
a very interesting trial involving patients with refractory advanced
breast cancer who were treated with very low doses of cyclophosphamide
and very low doses of weekly methotrexate, and some responses were
observed. These clinical studies offer evidence suggesting that
there might be something to this concept.
It could be argued that another mechanism of action for these
regimens is that daily oral cyclophosphamide is more likely to
induce ovarian dysfunction. I think, however, that chemotherapy
has cytotoxic effects in and of itself. Part of the effect of chemotherapy
in premenopausal women may be related to its effects on ovarian
function, but it’s certainly not the whole story.
SWOG-S0221: Use of growth factor support
Pegfilgrastim is used in the dose-dense arm of the new SWOG-S0221
trial because it certainly makes the regimen more acceptable to
patients. Looking at the time course to recovery of neutropenia,
it appears that administration every 14 days is possible. There
are anecdotal results indicating this is quite tolerable.
Initially, filgrastim will be used in the experimental arm of
weekly doxorubicin and daily oral cyclophosphamide. At the University
of Washington, pilot studies are being performed to evaluate pegfilgrastim
with this regimen. If those studies show that this combination
is safe, as expected, then we hope to amend the protocol and use
pegfilgrastim in both arms of the study.
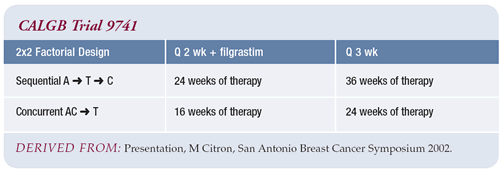
Results of CALGB-9741 trial
CALGB-9741 is a very interesting and provocative study, particularly
the 31 percent relative reduction in mortality. However, the results
are preliminary because it is very early in follow-up. At least
in the first few years, the results should be stable. Dr Piccart's
accompanying editorial was intriguing and laid the groundwork for
the trial we are launching.
Also, it was of interest that in CALGB-9741, there was no clear
difference between patients with ER-positive and ER-negative disease.
I feel comfortable offering this every-two-week regimen to patients
with ER-positive or ER-negative disease.
It is also interesting that these were not new agents being studied
in CALGB-9741. There is a lot to be learned using currently available
agents, and we can continue to take incremental steps in treating
breast cancer.
Impact of CALGB-9741 on clinical practice and
ongoing trials
On the practice level, physicians have been quite willing to
adopt dose-dense regimens, partly because there is no increase
in toxicity. In fact, in terms of neutropenic fever or documented
myelosuppression, the every-two-week regimen is less toxic. More
transfusions are required, but this is something we can deal with
if we monitor counts and start replacement therapy with erythropoietic
agents.
In terms of ongoing Phase III studies, the trials that I see
in need of modification are N9831 and other trastuzumab trials.
If a patient was randomized to the standard arm of doxorubicin/cyclophosphamide
given every three weeks and followed by a taxane, there would be
a nagging concern that the patient was not receiving optimal therapy.
I think there has to be strong consideration made to amend those
trials.
Managing patients with node-positive breast
cancer
In the nonprotocol setting, I feel obligated to discuss the results
from CALGB-9741 with patients who have positive nodes. After discussing
the fact that these were very early results but perhaps relevant
to a particular patient’s care, I have treated some patients
at high risk with this dose-dense regimen.
I also discuss standard treatment options, including the combination
of doxorubicin and cyclophosphamide followed by a taxane, although
I also discuss CAF-type regimens
Case discussion: Dose-dense chemotherapy for
locally advanced disease
This is a very intelligent woman in her late 40s, in whom locally
advanced breast cancer developed over a period of time. She was
quite panicked and very anxious to begin therapy, but at the same
time quite fearful of starting treatment. She was on vacation when
she first noticed the tumor, so there was a delay in seeking medical
care, and naturally she was quite concerned about the possible
result of this delay.
On physical examination, she had a breast mass on the left side
that was about 8-cm. There was some erythema over the tumor, although
it was not a true inflammatory breast cancer. There was palpable
adenopathy, which was not fixed. I think it was a relatively rapidly
growing tumor, but perhaps not the most aggressive that I’ve
seen. The hormone receptor and the HER2 status are currently pending.
I wanted to start her on treatment, but the locally advanced
study at our institution was not open. I discussed a variety of
treatment options with her. In my mind, the standard treatment
is an anthracycline-containing regimen, and I believe a taxane
should generally be used.
I presented the every-two-week doxorubicin/cyclophosphamide regimen
as an option, believing we could generalize the results from CALGB-9741
to her situation. We reached a mutual decision to embark on that
regimen and, as part of that, she is receiving pegfilgrastim.
A week after starting treatment, her tumor already seems to be
responding and surgery is planned. I had discussed giving her doxorubicin/
cyclophosphamide for four cycles followed by a taxane, which can
be given preoperatively or postoperatively. If an extensive reconstruction
(i.e., a TRAM reconstruction) is planned, there may be advantages
to giving the taxane prior to surgery, so there’s no delay
in administering the taxane.
Next page
Page 1 of 2
|
