| You
are here: Home: BCU 3|2003: Editor's
Note

 |
Editor’s Note |
|
| Two Women |
The plethora of “P” values and Kaplan-Meier curves
permeating the oncology research literature sometimes makes it easy
to forget that the building blocks of clinical trials are people
— doctors, nurses and most importantly, patients. In this
issue, Kathy Miller presents two women who participated in Phase
III randomized trials that had a fundamental impact on our understanding
of breast cancer treatment.
The first was a 62-year-old woman who enrolled in the ECOG-1193
trial. This classic trial, which was led by Kathy’s colleague
and mentor, George Sledge, addressed the critical question of combination
versus sequential chemotherapy in women with metastatic disease.
For years this study has been presented and mentioned at oncology
meetings, but the definitive paper was only recently published in
the February 15, 2003 issue of the Journal of Clinical Oncology.
While the paper concludes that sequential single-agent chemotherapy
results in the same overall survival as combination chemotherapy,
Kathy’s case reveals the challenges in incorporating data
from a patient’s individual course to a clinical trial.
This woman was randomized to the single-agent arm, and there was
essentially no response to the first agent (doxorubicin) and a modest
response to the crossover (paclitaxel), which also caused significant
toxicity. However, after the primary randomization, major longstanding
complete and near-complete responses were induced with anastrozole,
and then capecitabine. The patient eventually died from an unrelated
cerebrovascular event while experiencing excellent tumor control
from capecitabine. Dr Miller noted that while this woman’s
extended survival contributed to the single-agent randomization
arm of ECOG-1193, it was the post-trial therapy that seemed to have
the greatest effect in prolonging her life.
One can argue that large numbers of patients accrued to a study
will obviate outlying clinical events such as these, but any tumor
board meeting will provide more than adequate testimony to the heterogeneity
of breast cancer, particularly in the metastatic setting. Kathy’s
second case, presented at the 2002 San Antonio Breast Cancer Symposium
“Meet the Professor” session, demonstrates another critical
point about interpreting clinical research.
This 49-year-old woman was enrolled in a historic study —
the first major randomized breast cancer clinical trial evaluating
an antiangiogenic agent. Many attendees at the San Antonio Breast
Cancer Symposium were disappointed that this trial failed to demonstrate
its primary endpoint — a time to progression advantage for
the combination of capecitabine and bevacizumab compared to capecitabine
alone. In the interview for this program, Dr Miller presents the
provocative course of her patient with chest wall recurrence to
highlight the complexities of interpreting data from clinical trials
in patients with metastatic disease.
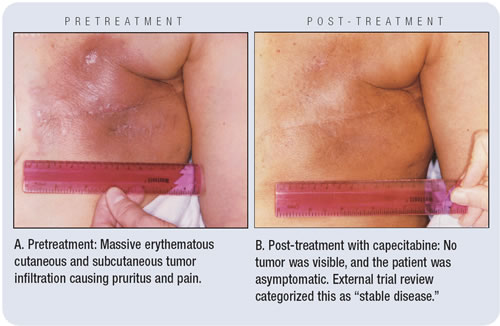
To Dr Miller’s eyes, this woman’s tumor had a rapid
and extremely impressive objective complete response to single-agent
capecitabine (see photos below), and the symptoms from her aggressive
tumor also completely abated. However, the external review board
— evaluating the photos and clinical notes — called
this “stable disease.” Kathy concedes that based on
the very conservative trial guidelines for external review, this
was a correct interpretation, but this case vividly portrays the
complexity of determining the antitumor effect of therapies in clinical
trials.
In an era of “evidence-based” medicine, clinicians
should consider that the foundation for clinical research is the
individual patient and that complex biopsychosocial variables make
clinical research a less exact science than laboratory investigation.
Ultimately, patients and physicians in daily practice routinely
confront a panoply of imperfect trial data that must be judiciously
evaluated in the context of each patient’s needs and values.
—Neil Love, MD
|
