| You
are here: Home: BCU 3|2003: Kathy
Miller, MD

Edited comments by Dr Miller
| CASE
1 62-year-old, postmenopausal woman with ER-positive, HER2-negative
metastatic disease |
| 1995: |
Modified radical mastectomy (node-negative),
adjuvant tamoxifen. |
| 1997: |
Pulmonary and hepatic metastases. Enrolled
in the ECOG-1193 trial and randomized to single-agent
doxorubicin. Received eight cycles with stable disease. |
| 1998: |
Paclitaxel crossover on the trial; partial
response. After nine cycles, progression to prestudy
status. |
| 1998: |
Treatment off-protocol with anastrozole:
Complete response. During the remission, patient had
breast reconstruction and contralateral breast reduction
for symmetry. |
| 2001: |
Bone metastases. Treatment with megestrol
acetate, a bisphosphonate and radiation therapy for hip
discomfort. |
| 2001: |
Rapid progression in pulmonary nodules.
Capecitabine administered with near complete response. |
| 2002: |
Death from cerebrovascular event unrelated
to the breast cancer. |
|
Treatment alternatives after progression on adjuvant
tamoxifen
If I were to treat this woman today, I would probably start with
hormone therapy, even with visceral disease. She’s asymptomatic,
has a tumor that is strongly ER-positive and her disease is easy
to follow with a simple chest x-ray. It would be reasonable to
give this woman additional hormonal therapy with an aromatase inhibitor
and repeat her chest x-ray in two or three months to evaluate response.
At this stage, hormonal therapy would be more beneficial than chemotherapy
in terms of quality of life, and I don’t think it would alter
her survival.
I’ve used fairly equal amounts of anastrozole and letrozole
in cases like this. I don’t know of any data directly comparing
them in practice, and their side effects are similar. We have first-line
trials with both agents comparing them to tamoxifen that show — depending
on the endpoint — that they are equivalent or superior. As
for the adjuvant setting, we don’t yet have any data on letrozole.
Breast reconstruction in patients with metastatic
disease
This was the first time I sent a patient with metastatic disease
for reconstruction. The plastic surgeon called and asked, “What
are you doing here? She has metastatic disease, so what’s
the difference?” And I responded, “She has been asking
me for this for quite a while now, and she’s still in complete
clinical remission. I don’t know how long this is going to
last, but it seems like it’s going to be a while. And when
it stops working, we’re going to switch to another hormone.” Reconstruction — even
with metastatic disease — seemed reasonable, because I hoped
she still had several years ahead of her. She fully recovered from
the reconstructive surgery and was absolutely delighted to be rid
of her prosthesis. She was a large-breasted woman and the inequity
in size and stress on her neck and back was difficult for her,
so she also had contralateral breast reduction.
Quality of life: Chemotherapy versus hormonal
therapy
Quality of life is significantly better for patients on hormonal
therapy than on chemotherapy. While this patient tolerated chemotherapy
quite well, she still felt pretty weak for several days after each
treatment. She experienced some fatigue and nausea, but she was
able to maintain her weight. She developed significant neuropathy
with the paclitaxel, which was beginning to interfere with her
activities at about the time her disease was progressing. She was
having difficulty manipulating buttons and holding a pen — certainly
not things that we would consider life-threatening toxicities,
but certainly lifealtering. She had been active with her church
and volunteer activities prior to treatment. Although she remained
functional on chemotherapy, it was to a lesser degree. She had
to discontinue all of her volunteer activities and didn’t
do much other than attend church. After she switched to hormonal
therapy, she was able to return to all of her previous activities.
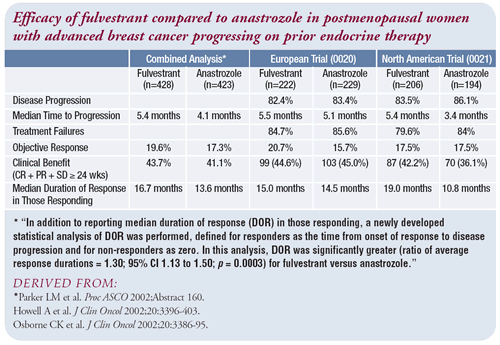
Hormonal therapy after progression on anastrozole
If fulvestrant had been available, I certainly would have considered
it. I am not aware of much data evaluating the response to fulvestrant
after aromatase inhibitors, but knowing the data after tamoxifen,
I expect there are patients who will respond. While the actual
response rates for patients progressing on tamoxifen were similar,
fulvestrant improved the duration of response by a couple of months
compared to anastrozole. That can be very important to a patient.
In the absence of data as to which treatment is going to give
the best or longest-lasting response, issues like convenience and
compliance become even more important and need to be discussed
with the patient. This patient was already being seen every four
weeks for her bisphosphonate infusion, so treatment with fulvestrant
would not have required extra trips to the clinic.
Some patients prefer receiving one injection a month, versus
having to remember to take a pill every day. I recently saw one
patient who admitted that she probably took only 10 percent of
her adjuvant tamoxifen because she just doesn’t like taking
pills and she forgets. When she was suspected of having metastatic
disease, she claims she “got religion” about her tamoxifen,
but still only managed to take it about 75 percent of the time.
Chemotherapy after progression in the asymptomatic
patient
After progressing on hormones for adjuvant therapy and metastatic
disease, single-agent capecitabine, paclitaxel or vinorelbine are
all reasonable choices for chemotherapy in this patient. The single-agent
response rates are similar, but they have different toxicities
and different modes of administration. Docetaxel and anthracyclines
are very active single agents, but overall their toxicities are
more difficult.
Capecitabine is a perfectly valid first-line option for an asymptomatic
patient who’s been on hormonal therapy for two or three years,
is used to taking pills and is not concerned about hair loss. It
eases patients from hormonal therapy into chemotherapy psychologically
and in terms of side effects.
Switching patients from pills to intravenous therapy can trigger
thoughts that they must be really sick and nearing the end. On
the other hand, some patients think pills are less effective, which
is not true, but we have to work with our patients’ perceptions.
Comorbidities are also a factor in selecting an agent. A diabetic
patient with peripheral neuropathy should avoid taxanes or vinorelbine
because of potential neuropathic toxicities. Paclitaxel and docetaxel
can be problematic for diabetics because of the need for premedication
with steroids. Vinorelbine may not be a good choice for women with
long histories of constipation.
Switching to a therapy that requires a vascular access device,
like a Hickman, is a much bigger step for the patient than for
the oncologist. Many patients see it as an end-stage measure, although
after they have it in, they are generally delighted with it. But
it’s a big step that many patients are not emotionally ready
to take, and you won’t be able to administer vinorelbine
for more than a few weeks without an access device.
Chemotherapy after progression in the symptomatic
patient
In the symptomatic patient with rapidly progressing metastatic
disease, treatment is aimed at getting the disease under control
in order to improve and prolong the patient’s quality of
life. In this situation, we need to shrink the cancer as quickly
as possible, with the hopes of then switching to either a less
toxic chemotherapy agent or dosing schedule, or to a hormonal therapy.
We have typically used a combination of an anthracycline and
a taxane, but the docetaxel/capecitabine combination is equally
reasonable. It’s difficult to compare the two combinations.
We know there’s a survival advantage with the docetaxel/capecitabine
combination, and there’s no survival advantage with an anthracycline/taxane
combination.
But that’s a bit of comparing apples and oranges because
many of the anthracycline/taxane combinations — including
the largest ECOG-1193 trial — included a crossover, and the
docetaxel/capecitabine trial didn’t. Had they done the study
using the ECOG-1193 model — combination versus each single
agent by itself with a crossover in the two single-agent groups — I
think they would have seen the same results as ECOG-1193: slightly
higher response rates with the combination, a minimal increase
in time to progression and no difference in overall survival or
quality of life.

The future of targeted therapies
We are moving towards more targeted treatments, but I’m
a little less optimistic about how quickly we will get there than
I was a few years ago. We hope one day to simply obtain a small
sample of the patient’s tumor, grind it up and put it on
a microarray, that will spit out 10,000 genes and tell us, “This
patient has breast cancer Type 15, which you treat with therapy
X, which has a 98 percent survival rate.” In order to reach
that point, we need a large volume of a wide variety of tumors
from patients who were treated in standard fashions, and then we
need to know what happened to them. Then we can start to identify
patterns of exquisite sensitivity or incredible resistance to a
particular agent. However, our ability to collect tumor samples
is a problem because so many of the patients are not at university
centers, where research on genomics and proteomics is being conducted.
Looking back at this case, we could not have predicted in advance
that this patient would have minimal response to doxorubicin and
paclitaxel and an excellent response to both anastrozole and capecitabine.
It would have been helpful to have known this information at the
onset.
If I had put her on anastrozole initially and switched her to
capecitabine when she progressed, she would have died of her stroke
without ever having had alopecia, nausea, myelosuppression or any
other associated toxicities.
| CASE
2 49-year-old school teacher with chest wall recurrence after
mastectomy, regional radiation and adjuvant chemotherapy |
Initial
diagnosis: 3-cm,
ER-negative, HER2-negative breast cancer with two positive
nodes. Treated with modified radical mastectomy, adjuvant
anthracycline/taxane regimen and chest wall radiation.
Nine months later: Chest
wall recurrence, no evidence of distant metastases. Enrolled
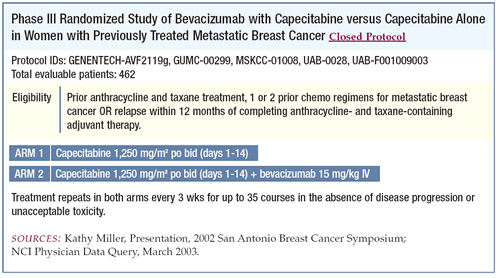
on single-agent capecitabine arm in a trial randomizing patients
to capecitabine with or without bevacizumab. Complete response
of tumor after two cycles of capecitabine (see page 5).
Seven months later: Second
primary breast cancer in contralateral breast, treated with
mastectomy. Capecitabine continued.
Four and a half months
later: Bilateral chest wall progression.
Vinorelbine administered with good response.
Some months later: Disease
progression. Since that time, the patient has been treated
in several Phase II trials, single-agent gemcitabine and
doxorubicin HCL liposome injection with minimal response.
|
Phase III trial of capecitabine with or without
bevacizumab in patients with previously treated metastatic breast
cancer
This trial looked at a very refractory group of patients, all
of whom had received an anthracycline and a taxane. This patient
was in the subset of women who had received these drugs as adjuvant
therapy and had not received any therapy for metastatic disease.
We considered designing the trial with a crossover to the bevacizumab
arm but didn’t for pragmatic reasons. The trial was designed
with progressionfree survival as its primary endpoint, but overall
survival was a secondary endpoint.
Efficacy of capecitabine with and without bevacizumab
The efficacy data confirmed the activity of bevacizumab reported
in the Phase II trial with a similar patient population. There
was a near doubling of response rates in patients receiving the
combination of bevacizumab and capecitabine versus capecitabine
alone.
The trial enrolled 462 patients and responses were assessed by
an independent review facility, as well as by investigators. There
is generally a discrepancy between these two groups. Some describe
the independent review facility as more objective, while others
say it is more cynical.
In this study, the independent review facility reported a lower
response rate than the treating physicians. Although the combination
therapy increased the response rate, most of the additional responses
were short-lived. Therefore, the proportion of long-term responders
and the progression-free survival was the same in both groups.
Next page
Page 1 of 2
|
