| You
are here: Home: BCU 3|2003: Kathy
Miller, MD

Tolerability and side-effect profile of capecitabine/bevacizumab
The toxicity data were reassuring. The capecitabine toxicities
were similar to what was already reported in the literature, and
the bevacizumab toxicities were as expected based on the Phase
II results. About 20 percent of patients experienced hypertension
requiring intervention. Ten to 20 percent of patients experienced
proteinuria, although rarely severe, and no Grade IV events were
reported.
Grades I and II bleeding were slightly increased, but Grade III
bleeding was extremely uncommon and not increased by the addition
of the antiangiogenic agent. There was a slight increase in thrombosis,
predominantly deep vein thrombosis and line-associated thrombosis,
but no increase in serious thrombotic events or pulmonary embolism
with the combination.
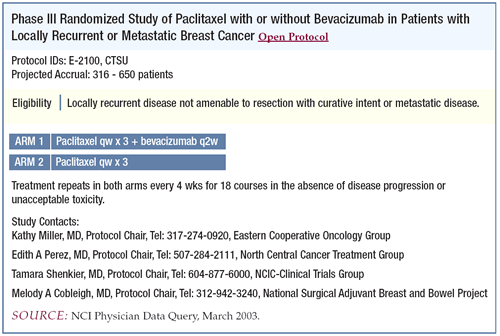
ECOG-E2100 trial: Phase III trial of paclitaxel
with or without bevacizumab in patients with previously untreated
metastatic breast cancer
This study takes bevacizumab to the next step. It moves the use
of this agent to newly diagnosed, locally recurrent or metastatic
patients and combines it with paclitaxel — a combination
for which we have a lot of preclinical synergy data.
Bevacizumab is a targeted therapy, yet we currently treat patients
based on the history of their disease rather than molecular factors.
We need to determine how to select the patients most likely to
respond to this type of therapy, but we don’t know which
factors are going to be predictive.
This study is prospectively collecting primary tumor tissue,
serum and urine samples for investigation of potential surrogates
of response to VEGF targeted therapies. We hope VEGF is predictive,
but it’s technically difficult to measure and be certain
that what you’re measuring reflects the tumor, and not the
white blood cells, macrophages and platelets.
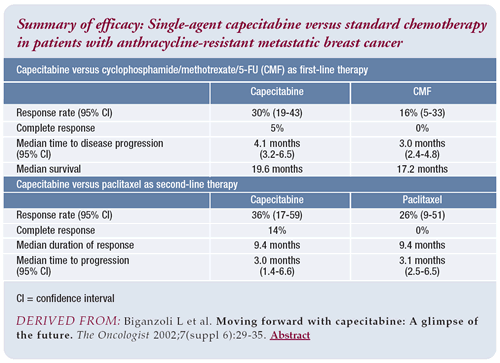
Single-agent capecitabine for metastatic breast
cancer
When discussing the trial, I told this patient that capecitabine
was one of the best therapies for her disease and that if she was
randomized to the capecitabine-alone arm, she would receive exactly
what I would have given her if the trial was not a possibility
or didn’t exist. She was disappointed when she first learned
she was not randomized to the investigational arm, but she has
a wonderful, supportive spouse who reminded her that they knew
when they agreed to enter the trial that this might happen, and
she was still receiving the best I had to offer. That quickly turned
her around and she was willing to continue with the study.
It would have been reasonable to consider other single agents,
such as vinorelbine or gemcitabine, but even off-study I believed
that capecitabine was the best choice for her. She did not want
to miss teaching days, so oral therapy was a real advantage and
she didn’t have alopecia. She tolerated the treatment very
well. She had a dose reduction for Grade II hand-foot syndrome
after her first cycle of therapy, and she did not require any other
dose modifications.
After two cycles of capecitabine, she had a complete response
with all visible and palpable evidence of her disease completely
gone. Her chest wall discomfort disappeared as well, and she was
completely symptom-free. It was an amazing response — minimal
toxicity, symptoms gone, she felt well and she was able to continue
teaching.

Selecting a taxane schedule for progression following
adjuvant taxane therapy
Phase II data in patients with metastatic disease who received
an adjuvant taxane show that you can switch to another taxane or
use the same taxane on a different schedule and still obtain some
additional response. I have certainly done that, but when considering
the alternatives, it’s not my first choice. Not because there’s
data that says it wouldn’t work — it just doesn’t
feel right. It’s an agent the patient already tried, it didn’t
produce the desired results and I have a number of other options.
Typically, I will discuss it with patients and tell them that at
some point we’re likely to come back to the taxanes, but
I prefer to try other therapies first.
Discrepancies between the findings of investigators
and an independent review facility
The capecitabine with or without bevacizumab trial included a
detailed process for reporting findings to an independent review
facility. Chest, abdominal and pelvic CTs were taken at every assessment
point and sent to the reviewers, regardless of the patient’s
status. All sites had the same type of Polaroid cameras to take
photos of skin lesions with rulers in the image. And all of the
physicians’ clinical notes were sent after they were censored
to ensure they did not indicate to which treatment group the patient
was assigned.
Still, as we’ve seen in other trials, the independent review
facility rated responses eight to ten percent lower than the investigators.
Part of that is probably realistic — some of the patients
we say are responding probably don’t quite meet the strict
criteria set forth by the study — but part of that is the
difficulty of assessing a patient whom you cannot examine.
This patient was one for whom there was significant discrepancy
between the independent reviewers’ and the investigators’ assessments.
When this patient had a complete response following capecitabine,
the independent review facility reported her as stable. I would
have done the same if I had been part of the independent review
facility. While they have my physical exam notes telling them the
chest wall is no longer indurated and the nodules are gone, they
have no way to independently verify that. They use the clinical
data to document progression, but regression on physical exam can’t
be verified, so the best they can do is call it stable disease.
In addition, they have to rely on photos that may not document
skin changes well, particularly in darker-skinned women. This Hispanic
patient had an intense hyperpigmentation following radiation. When
her disease progressed, the skin lesions covered the radiation
field and extended beyond it. Even though those lesions disappeared
following two cycles of capecitabine, the hyperpigmentation from
radiation was still visible. So, the independent review facility
could only look at those photos and say, “This is not normal,” and
label her disease as stable while we reported a complete response.
Treatment of a second primary and progression
in advanced breast cancer
This patient had a very interesting course. She continued on
capecitabine but came off study for progression, which was actually
a second primary breast cancer without recurrence of the previous
extensive chest wall disease. We repeated all of her systemic staging,
and there was absolutely nothing. She had a modified radical mastectomy
and continued the capecitabine.
Four and a half months later; her cancer recurred with bilateral
chest wall involvement that was actually worse on the side that
was not radiated. Whether it was recurrence of two primary tumors,
I don’t know, but it was a curious pattern. She was treated
with single-agent vinorelbine and had an excellent response.
Palliation of persistent, localized breast cancer
I’ve had a couple of patients with persistent, localized
breast cancer, which can be intensely painful and socially isolating.
It’s difficult to put on normal clothes when there’s
a weeping, sometimes bleeding, often super-infected wound wrapping
around one’s chest. I’ve had several patients who don’t
want to be around people because they’re self-conscious about
the offensive odor.
Pain control is also very difficult. This patient didn’t
like the grogginess she experienced with narcotics, and the drugs
we use for neuropathic discomfort didn’t help much. We tried
some topical anesthetics, but as the disease became more extensive,
there was a problem with systemic absorption, so we’re not
able to use those anymore. Women with this type of localized disease
tend not to have a lot of visceral disease. It’s certainly
not the quality of survival for which we strive.
Quality of life and continued treatment for advanced
disease
This is a truly amazing woman and I’m honored to care for
her. She knows of my reservations regarding further treatment because
we’ve discussed it several times. Yet, when I last saw her
two weeks ago, the question utmost in her mind was, “How
can you get me back to the classroom, because I need to finish
out the year?” All she ever wanted to do was teach. She’s
taught me an incredible amount, and she’s still a teacher
whether she’s in a classroom or not.
She is a very strong-willed woman who would probably rate her
quality of life higher than I would at this point. She typically
refuses to take analgesics and I’ve had to adjust how I ask
her about pain because she usually says, “It’s okay.” Now
I go one step further and ask, “Would other people say the
pain medication is working well enough?” And then she’ll
admit that maybe it could be better. So, we continue adjusting
doses and schedules of her treatments so that she can to be in
the classroom as much as possible, because that’s what’s
most important to her.
This is a difficult situation. I can certainly come up with agents
that she’s not received, but we’re likely to have diminishing
returns with increasing toxicity, and I’m concerned that
I’ve made her feel worse, not better. I’ve tried very
hard to get her to think about not having additional chemotherapy,
but she is not quite ready to make that transition. In this situation,
I think her opinion still trumps mine.
Select publications
Return
Page 2 of 2
|
