| You are here: Home: BCU 5|2002: George W Sledge, Jr, MD
 |
 |
 |
 |
 |
George
W Sledge, Jr, MD |
 |
 |
Professor of Medicine & Pathology
Ballve-Lantero Professor of Oncology
Indiana University School of Medicine
Vice Chairman, Eastern Cooperative Oncology
Group Breast Cancer Committee
Member, FDA Oncology Drug Advisory Committee
Member, Department of Defense Breast Cancer
Research Program Integration Panel |
|
 |
 |
 |
Edited comments by Dr Sledge
Cardiac effects of trastuzumab
When given in combination with an anthracycline, trastuzumab significantly
increases the risk of congestive cardiomyopathy. In the pivotal
trial by Slamon et al, the group of women receiving trastuzumab/paclitaxel
after a prior anthracycline-containing regimen experienced a smaller
yet real risk of cardiac events, including an occasional case of
congestive heart failure.
Since anthracyclines may be more effective in women with HER2-positive
breast cancer, they are frequently used in this patient population.
In 1998, ECOG designed trial E2198 to evaluate these safety issues.
Women with HER2-positive (IHC 2+ or 3+), node-positive breast cancer
were given four cycles of paclitaxel/trastuzumab followed by four
cycles of doxorubicin/ cyclophosphamide and then randomized to either
stop therapy or receive trastuzumab. The hypothesis, at that time,
was that with this schedule, the interaction between trastuzumab
and doxorubicin would not occur. Hence, there would be a lower incidence
of congestive cardiomyopathy.
However, we have since learned from Dr Brian Leyland-Jones that
trastuzumab has a longer half-life than once thought. Although we
designed the trial for patients to have discontinued trastuzumab
three weeks before receiving doxorubicin, we now know that three
weeks is not enough time for trastuzumab to be eliminated from the
patient’s body.
It is likely that the patients still had circulating trastuzumab
in their blood when they received doxorubicin. With that in mind,
the results are still reassuring. We assumed that the baseline incidence
of congestive heart failure was slightly less than 1%. In our trial,
there was a 1.7% incidence of drug-related congestive heart failure.
After receiving trastuzumab/paclitaxel, a few patients had a temporary
decline in their left ventricular ejection fractions, which resolved
despite subsequent treatment with doxorubicin and cyclophosphamide.
There are certain patients in whom one would want to avoid four
cycles of trastuzumab/paclitaxel followed by four cycles of doxorubicin/cyclophosphamide.
Virtually all of the patients who had cardiac problems in our trial
were predisposed to cardiac disease.

Trastuzumab for patients with metastatic breast
cancer
HER2-positive, metastatic breast cancer is a life-or-death situation
and therefore quite different than the adjuvant setting. As observed
in the ECOG trial and the pivotal trial by Slamon et al, the average
survival for these patients when treated with standard chemotherapy
was about 17 months, and trastuzumab clearly improved survival.
Even though 25% of the patients receiving trastuzumab plus an anthracycline
developed a cardiac event, trastuzumab still improved survival in
that group. It is reasonable to use trastuzumab in the vast majority
of patients with HER2-positive, metastatic breast cancer.
I routinely use trastuzumab as part of my first-line therapy for
patients with HER2-positive, metastatic breast cancer. Whether to
use trastuzumab alone or in combination with chemotherapy is a separate
question. In patients with an impaired performance status, it would
be reasonable and appropriate to give trastuzumab alone. My sense
is that the majority of community oncologists are using trastuzumab
in combination with chemotherapy as first-line therapy for HER2-positive,
metastatic breast cancer. Over the last couple of years, there has
been a trend to use trastuzumab earlier in the metastatic setting.
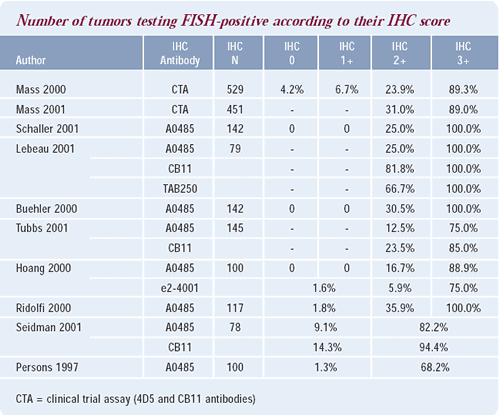
Algorithm for assessing HER2 status
Patients with tumors that score 2+ on immunohistochemistry (IHC)
are frequently found to be HER2-negative when tested by fluorescence
in situ hybridization (FISH). In those patients, I routinely have
their tumors retested by FISH. On the other hand, I do not obtain
a FISH analysis for patients whose tumors score 3+ on IHC from a
laboratory where I trust the pathologist.
Since HER2-positive breast cancer has a fairly specific phenotype
(i.e., steroid receptor-negative, younger age, early relapse), I
will retest those types of patients by FISH if I have a two- to
three-year-old IHC score of 0 or 1+. If the patient’s tumor
is IHC-negative and FISH-positive, I will treat them with trastuzumab
despite the fact that we do not have clinical data for that group
of patients. Tumors that are FISH-positive are likely to have ample
amounts of HER2 receptors on their cell surface.
We lack quality control for both IHC and FISH. This is analogous
to the situation encountered with estrogen receptors in the mid-
to late 1970s. I suspect HER2 testing in the year 2001 was very
similar to estrogen receptor testing in the year 1975 or 1976. One
wonders how many patients died because they did not receive adjuvant
tamoxifen as a result of inadequate estrogen receptor testing. If
adjuvant trastuzumab provides a benefit like adjuvant tamoxifen,
we may encounter exactly the same problem.
Continue
Page 1 of 2
|
