| You are here: Home: BCU 5|2002: George W Sledge, Jr, MD
ATAC trial results
Albeit with very early follow-up, the ATAC trial suggests for
the first time that an aromatase inhibitor — anastrozole —
might be superior to adjuvant tamoxifen. Fascinatingly, the combination
of tamoxifen and anastrozole was no better than tamoxifen. There
was a marked reduction in the development of contralateral breast
cancers in patients receiving adjuvant anastrozole compared to adjuvant
tamoxifen. We already knew adjuvant tamoxifen reduced the risk of
contralateral breast cancers by up to 50%. Adjuvant anastrozole
provided a significant additional benefit on top of that associated
with adjuvant tamoxifen. The difference between anastrozole and
tamoxifen was striking.
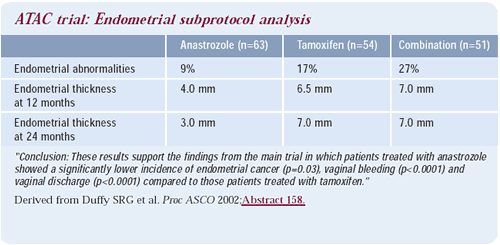
In the ATAC trial, there also appeared to be a lower risk of deep
vein thrombosis, endometrial cancer and hot flashes associated with
anastrozole than with tamoxifen. From a toxicity standpoint, anastrozole
may be better tolerated than tamoxifen. These results represent
a fascinating new avenue in terms of therapy, not only in the adjuvant
setting, but also in the chemoprevention setting.
Premalignant breast changes in the NSABP P-1
trial (tamoxifen versus placebo)
The NSABP looked at the incidence of premalignant changes in the
breast, such as cystic disease, hyperplasias (typical and atypical)
and fibroadenomas. In women receiving tamoxifen as a chemopreventative
agent, there was a generalized reduction in premalignant changes
of the breast, which was age-related. There was a huge reduction
in the premalignant and the nonpremalignant events for younger women
and little or no reduction in many of the events for older women.
From a toxicity standpoint, tamoxifen is associated with thromboembolic
events and endometrial cancer primarily in older women. Therefore,
tamoxifen as a chemopreventative agent is more appealing in younger
women.
Adjuvant ovarian ablation/tamoxifen
For a long time, American oncologists have believed that hormonal
therapy was of secondary importance in the treatment of breast cancer
and that aggressive doses of chemotherapy were more imperative.
However, the kinder and gentler approach aimed at the biology of
the tumor is also very important. The better we are at shutting
off the estrogen receptor pathway, the better the disease-free and
overall survival for our patients.
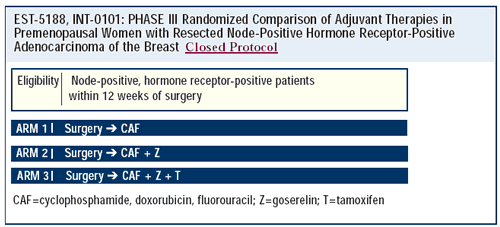
In the late 1980s, an Intergroup trial (INT-0101) was designed
to evaluate the role of adjuvant ovarian ablation with or without
tamoxifen. It randomized premenopausal women with node-positive,
estrogen receptor-positive breast cancer to CAF (the standard chemotherapy
at the time), CAF followed by goserelin or CAF followed by goserelin/tamoxifen.
Since INT-0101 was designed at a time when tamoxifen was not thought
to benefit premenopausal women, an arm consisting of CAF plus tamoxifen
was not included. Goserelin had the greatest effect in the youngest
group of women — those under the age of 40. This implies that
shutting off a woman’s ovaries, when chemotherapy has not
already done so, is a good thing.
In my own practice, I have tended to offer more ovarian ablation
to younger women than I did three or four years ago. The most effective
way to reduce the risk of recurrence in premenopausal women is to
deprive them of estrogen. In the group of women randomized to CAF,
those who became menopausal had a better outcome than the women
who did not experience menopause as a result of chemotherapy. There
are two plausible explanations for this finding. Either estrogen
deprivation related to menopause is beneficial or the patients who
became menopausal did so because they had higher CAF serum concentrations.
Therefore, menopausal status may simply be a marker of the higher
blood levels of cytoxic agents.
Management of node-positive, ER-positive patients
who do not become menopausal after adjuvant chemotherapy
In some patients, I recommend either surgical or medical ovarian
ablation. It would also be reasonable to utilize tamoxifen. In INT-0101,
patients who received CAF followed by goserelin/tamoxifen did the
best. When inducing premature menopause, it is important to maintain
the patient’s general health. Therefore, one must pay attention
to the serum lipids and bone mineral density. Whether the addition
of an aromatase inhibitor will provide the maximum benefit to an
LHRH agonist needs to be evaluated in clinical trials.
Anti-vascular endothelial growth factor (VEGF)
monoclonal antibody
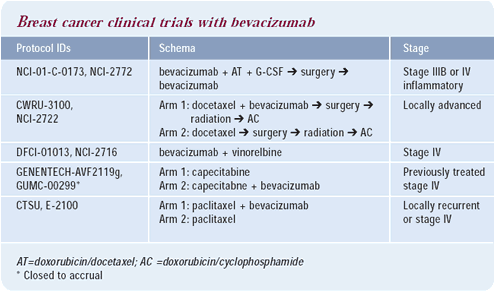
An upcoming ECOG trial, chaired by Dr Kathy Miller, will randomize
patients with metastatic breast cancer to weekly paclitaxel with
or without a recombinant humanized monoclonal antibody to vascular
endothelial growth factor (rhuMAb-VEGF, bevacizumab). This is the
first large trial evaluating an antiangiogenic agent as front-line
therapy in metastatic breast cancer. In anthracycline and taxane
refractory breast cancer, a trial evaluating capecitabine with or
without bevacizumab was recently completed.
In preclinical models, the taxanes have demonstrated antiangiogenic
activity. The taxanes kill vascular endothelial cells, affect capillary
tubule formation and affect capillary migration. Hopefully, combining
an antiangiogenic agent, such as bevacizumab, with a taxane will
lead to an additive or synergistic effect. Indeed, in some preclinical
models, there was a synergistic effect against endothelial cells
when a taxane was combined with anti-VEGF antibodies. Similarly,
there is preclinical data suggesting that capecitabine may have
some antiangiogenic activity. In the next few years, we will know
if antiangiogenic agents contribute significantly to the management
of breast cancer.
Select Publications
Page 2 of 2
|
