|
You are here: Home: BCU 5|2002: Anthony Howell, BSc, MBBS, MSc, FRCP
Edited comments by Dr Howell
Lack of benefit from anastrozole/tamoxifen compared
to anastrozole in the ATAC trial
I believe these findings were entirely predictable. In animal
models, such as the immature rat uterus assay, tamoxifen stimulates
uterine growth in a low estrogen environment. In a high estrogen
environment, tamoxifen acts as an antiestrogen. The lack of benefit
from the combination arm may be related to tamoxifen's action as
an agonist rather than an antagonist in a low estrogen environment.
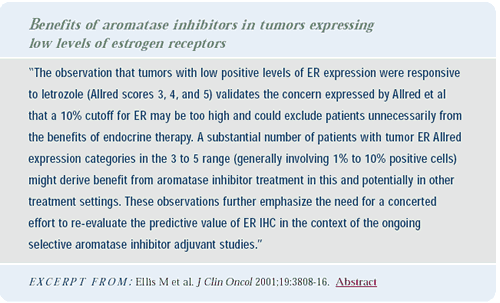
A potential explanation for the superiority of anastrozole is
suggested by data from Matt Ellis demonstrating that tamoxifen does
not seem to be as active as the aromatase inhibitors in low estrogen
receptor conditions. In his data, which revealed a correlation between
response rates and Allred's estrogen-receptor score, tamoxifen only
works with Allred scores above three; whereas, letrozole worked
with an Allred score of 3 and 4, as well as the higher scores. That
may be another reason why tamoxifen is not as effective as anastrozole.
This data may explain why one set of drugs — the aromatase
inhibitors — are better than another set of drugs, the SERMs.
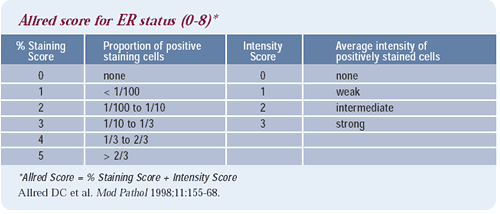
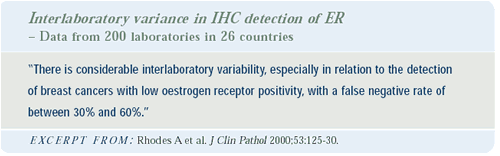
Defining ER positivity
There is variation in defining estrogen receptor positivity in
Europe and across the United States. I agree with Kent Osborne that
this variation is extraordinarily disturbing — particularly
as our hormonal therapies continue to improve. My feeling is that
if there is any receptor present in a tumor, it should be considered
positive. Clearly, we can miss a very low positive result quite
easily, and the result may be that patients who should receive adjuvant
endocrine therapy are not receiving it. We need to get this assay
correct for every woman.
Approximately 8% of the women in the ATAC trial had ER-negative
breast cancer, and there are women whose estrogen receptor status
is still unknown. More than 80% of the women in the ATAC trial had
ER- and/or PR-positive tumors, which is reassuring. Even if we include
the patients with estrogen receptor-negative and unknown disease,
there is still a statistical benefit in terms of disease-free survival.
There is a benefit in the intent-to-treat analysis of all patients.
Overall, anastrozole has an 18% reduction in the recurrence rate
compared to tamoxifen. This increases to 23% for those with known
ER positive disease.

Implications of the ATAC trial in chemoprevention
The 80% reduction in contralateral tumors in the anastrozole arm
of the ATAC trial is very exciting. If this translates into preventive
therapy, it augurs well for the future prevention of breast cancer.
The IBIS-2 prevention trial is in the planning stages, but it may
be a three-arm study involving anastrozole, tamoxifen and placebo.
We are also discussing the use of a bisphosphonate in IBIS-2. We
have so much data on clodronate, and it has a favorable side-effect
profile. Therefore, it may be the bisphosphonate of choice for the
trial. We plan to begin the study in September or October, and there
is enormous enthusiasm for this trial from investigators around
the world.
Impact of anastrozole on bone density: Potential
role of bisphosphonates
Overall, anastrozole has a much better toxicity profile than tamoxifen.
The risks of thromboembolic complications and endometrial cancer
are much lower, which makes it an attractive drug. One of the concerns
about anastrozole is how to address its potential effects on bone
density.
There was an increase in the fracture rate in the anastrozole
arm of the ATAC trial. Women in the control arm were given tamoxifen,
which prevents fractures; therefore, the increase in the fracture
rate with anastrozole was probably not as big as it looks. We estimate
that approximately half of the fracture rate in the ATAC trial was
due to the negative effects of anastrozole. In terms of monitoring,
I think most patients on anastrozole will need serial bone density
measurements.
Given the data from Ingo Diel and Trevor Powles on bisphosophonates
in the adjuvant setting, in the future we might be treating elderly
women with ERpositive breast cancer with a combination of clodronate
and anastrozole. Bisphosphonates may not only make anastrozole safe
with regard to bone density, they also have an effect on survival.
Clodronate is not available commercially in the United States, but
many osteoporosis researchers do not believe there is much difference
between the bisphosphonates. We need to find the bisphosphonate
with the lowest GI toxicity.

Continue
Page 1 of 2
|
|
