| You are here: Home: BCU 5|2002: Anthony Howell, BSc, MBBS, MSc, FRCP
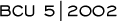
Fulvestrant and sequencing of endocrine therapy
Fulvestrant is a highly potent, estrogen receptor downregulator, which is equivalent as second-line therapy to our best drugs — the aromatase inhibitors. We now have another best drug. Now, women and physicians have a choice between treatments that are clearly equivalent.
New therapies for advanced breast cancer are useful, because we give endocrine agents sequentially. I believe that the first-line treatment for advanced disease in postmenopausal women — even those who have not had adjuvant tamoxifen — is an aromatase inhibitor. At the moment, I see fulvestrant being used after aromatase inhibitors in women who have not received an aromatase inhibitor in the adjuvant setting. It probably does not matter in which order you give them, but we have more data on aromatase inhibitors than fulvestrant.
There is a biological reason why fulvestrant might be better than anastrozole. Anastrozole lowers the serum estradiol levels, but there is still some estradiol present that could potentially stimulate the tumor. Fulvestrant blocks the receptor continuously; thereby preventing stimulation by circulating estradiol.
I do not believe that the fulvestrant injection is a problem. There have not been major problems with injection site reactions. In fact, it could be seen as an advantage, in that women would not have to take pills every day. I do not think women mind an injection if they are receiving an active compound.
Fulvestrant: Mechanism of action
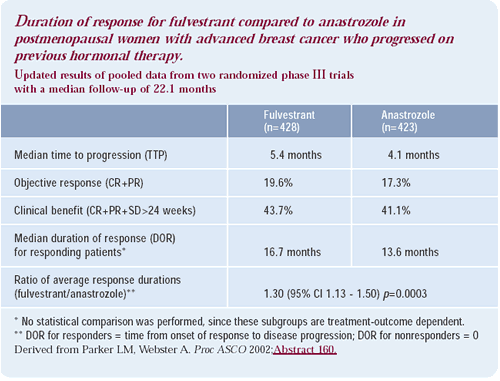
Tamoxifen and fulvestrant interact differently with the estrogen receptor. Tamoxifen causes receptor dimerization — binding to the estrogen response element — and activation of AF-1 but inactivation of AF-2. This causes partial estrogen-agonistic and partial antagonistic activity, depending on the cell and the gene promoter contact. In contrast, fulvestrant inactivates both AF-1 and AF-2, completely switching off the receptor, and it increases the turnover of the receptors themselves.
These effects were seen in the preoperative studies, where fulvestrant or tamoxifen was given, and tumor immunostaining for estrogen receptors was examined over a period of two to three weeks. After two weeks, almost as much estrogen receptor in the tamoxifen-treated group stained positive as in the pretreatment sample. In the fulvestrant arm, there was virtually no receptor to be stained.
In vitro effects of complete estrogen blockade
Dick Santen studied MCF-7 cells, which “hunt” prevailing estrogen concentrations. In vitro, MCF-7 cells are maximally stimulated by 10–9 molar units of estradiol. If these same MCF-7 cells are deprived of estrogen for one month, the dose response curve shifts dramatically to the left, becoming sensitive to 10–15 molar units of estradiol. The cells increase their sensitivity quite dramatically.
Estradiol levels are virtually undetectable in women on anastrozole. If Santen’s data is transferable to humans, there is the potential for the tumor to adapt to the lower prevailing estradiol concentration, thus circumventing the effect of anastrozole. This data may indicate a tumor’s ability to be stimulated by the low concentrations of estrogen that exist in patients treated with anastrozole.
This data from Santen is also very important for the future of endocrine therapy. For women who fail anastrozole therapy, we may be able to add back small doses of estrogen to cause tumor suppression — like high-dose estrogen in the “old days” but with the sensitivity curves shifted to the left.
We studied women who had four endocrine therapies, adding back estradiol in large doses. In this group of 30 patients, one-third responded for a median duration of one year. Thus, we may be able to rechallenge with estradiol and alternate aromatase inhibitors with estrogens. We have not sufficiently thought out endocrine therapy, and there may be more mileage here than we actually know.
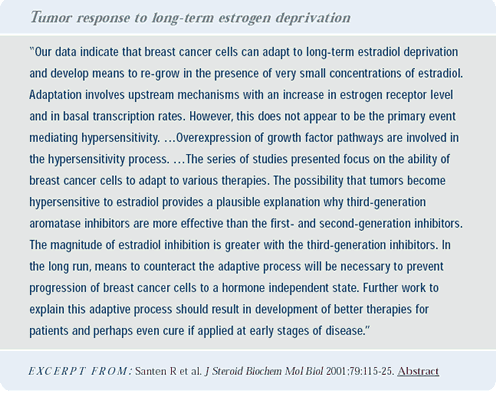
Preoperative fulvestrant
The preoperative EORTC trial evaluates one injection of fulvestrant after the diagnosis of breast cancer but before surgery. The idea is for the fulvestrant injection to cover the operative period as a potent antiestrogen that will lower estrogen receptor levels. We want to test the hypothesis of Bernie Fisher and others that adverse events related to metastases occur during the perioperative period. Hopefully, we can alter that with fulvestrant. The aim is to enroll more than 3,000 women into this study.
Select Publications
Page 2 of 2
|
