|

| Tracks 1-18 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Overview of recent results of
adjuvant trastuzumab trials |
| Track 3 |
Efficacy of trastuzumab/
carboplatin/docetaxel TCH arm
in BCIRG 006 |
| Track 4 |
BCIRG 006: Cardiac safety data |
| Track 5 |
Selecting an adjuvant regimen
for patients with HER2-positive
disease |
| Track 6 |
Controversies in HER2 testing |
| Track 7 |
Trastuzumab monotherapy in the
adjuvant setting |
| Track 8 |
Duration and sequencing of
adjuvant trastuzumab therapy |
| Track 9 |
Delayed adjuvant trastuzumab |
| Track 10 |
Predictors of response to adjuvant
trastuzumab |
|
| Track 11 |
Management of patients who
progress following adjuvant
trastuzumab |
| Track 12 |
Combining adjuvant trastuzumab
with hormonal therapy |
| Track 13 |
Combining trastuzumab with
chemotherapy in the metastatic
setting |
| Track 14 |
Selecting a chemotherapy
regimen to combine with
trastuzumab |
| Track 15 |
Combining trastuzumab and
bevacizumab |
| Track 16 |
CNS metastases in patients
receiving adjuvant trastuzumab |
| Track 17 |
Research strategies with lapatinib |
| Track 18 |
Perspectives on neoadjuvant
trastuzumab |
|
|
Select Excerpts from the Interview
 Tracks 3-4 Tracks 3-4
 DR LOVE: Can you summarize the recently released efficacy data from
the BCIRG 006 adjuvant trastuzumab trial? DR LOVE: Can you summarize the recently released efficacy data from
the BCIRG 006 adjuvant trastuzumab trial? |
 DR SLAMON: The efficacy data are based on the first interim analysis of a
three-arm trial with 300 events, and we recognize we’re walking a fine line,
but even so, both arms crossed their efficacy boundaries. We’ve known all
along that trastuzumab was the critical molecule. The relevant question is:
How does the TCH arm — the nonanthracycline arm — look relative to the
anthracycline-containing arm? We have a lot of safety information now, and
that’s got to be weighed against the efficacy information. DR SLAMON: The efficacy data are based on the first interim analysis of a
three-arm trial with 300 events, and we recognize we’re walking a fine line,
but even so, both arms crossed their efficacy boundaries. We’ve known all
along that trastuzumab was the critical molecule. The relevant question is:
How does the TCH arm — the nonanthracycline arm — look relative to the
anthracycline-containing arm? We have a lot of safety information now, and
that’s got to be weighed against the efficacy information.
What we can say now is that the risk reduction in the TCH arm is 0.39 (2.1).
The risk reduction in the AC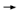 TH arm is 0.51, which is almost identical to
what was seen in the trials reported at ASCO for that kind of combination
(Perez 2005; Piccart-Gebhart 2005a; Romond 2005a). There are very few
event differences between the two trastuzumab arms. The two confidence
intervals completely overlap, and the statisticians have said there is no statistical
difference between the two arms. TH arm is 0.51, which is almost identical to
what was seen in the trials reported at ASCO for that kind of combination
(Perez 2005; Piccart-Gebhart 2005a; Romond 2005a). There are very few
event differences between the two trastuzumab arms. The two confidence
intervals completely overlap, and the statisticians have said there is no statistical
difference between the two arms.
 DR LOVE: Oncologists have to make practical decisions with the information
available. Based on these numbers, many may be thinking TCH will not be as
effective as the anthracycline arm. What are your thoughts on that conclusion? DR LOVE: Oncologists have to make practical decisions with the information
available. Based on these numbers, many may be thinking TCH will not be as
effective as the anthracycline arm. What are your thoughts on that conclusion?
 DR SLAMON: My thoughts are consistent with what the data show: There
is a numerical difference and it’s statistically insignificant. We need to wait
until the data mature, and it’s not going to take a long period of time. Physicians
should do what they feel most comfortable with at this point. If they feel
more comfortable with the AC DR SLAMON: My thoughts are consistent with what the data show: There
is a numerical difference and it’s statistically insignificant. We need to wait
until the data mature, and it’s not going to take a long period of time. Physicians
should do what they feel most comfortable with at this point. If they feel
more comfortable with the AC TH data, based on those relative differences
despite the fact they are not different statistically, they should go with that
arm, recognizing what everyone has said all along: Those patients are going to
have to be watched very closely for cardiotoxicity. TH data, based on those relative differences
despite the fact they are not different statistically, they should go with that
arm, recognizing what everyone has said all along: Those patients are going to
have to be watched very closely for cardiotoxicity.
 DR LOVE: What is the statistical likelihood that the TCH arm will be superior
to the AC DR LOVE: What is the statistical likelihood that the TCH arm will be superior
to the AC TH arm? TH arm?
 DR SLAMON: I have no idea at this point. All I can tell you, without giving
away information that will be presented at San Antonio, is that the results are
based on very few event differences. DR SLAMON: I have no idea at this point. All I can tell you, without giving
away information that will be presented at San Antonio, is that the results are
based on very few event differences.
 DR LOVE: Do you think adjuvant TCH is a reasonable alternative in the
clinical setting at this time? DR LOVE: Do you think adjuvant TCH is a reasonable alternative in the
clinical setting at this time? |
 DR SLAMON: Based on what we know, yes. TCH has been around a while
in the metastatic setting, and a lot of data have been presented, even randomized
data. DR SLAMON: Based on what we know, yes. TCH has been around a while
in the metastatic setting, and a lot of data have been presented, even randomized
data.
 DR LOVE: Do you have an adjuvant protocol available to you right now? DR LOVE: Do you have an adjuvant protocol available to you right now?
 DR SLAMON: We do not. DR SLAMON: We do not.
 DR LOVE: When you see a younger patient with a node-positive breast
cancer, which adjuvant therapies do you consider and what’s your usual
recommendation? DR LOVE: When you see a younger patient with a node-positive breast
cancer, which adjuvant therapies do you consider and what’s your usual
recommendation?
 DR SLAMON: At this point we try, whenever possible, to avoid anthracycline-containing
regimens because of the known interaction of trastuzumab with
anthracyclines. However, we’re not restricted to TCH. There are a number of
different drugs that interact very well with trastuzumab, including vinorelbine,
for which we have published data in the metastatic setting (Burstein 2003). DR SLAMON: At this point we try, whenever possible, to avoid anthracycline-containing
regimens because of the known interaction of trastuzumab with
anthracyclines. However, we’re not restricted to TCH. There are a number of
different drugs that interact very well with trastuzumab, including vinorelbine,
for which we have published data in the metastatic setting (Burstein 2003).
If the trastuzumab story has told us anything, it’s that what we see in the
metastatic setting gets even better in the adjuvant setting when we take it
forward. I know oncologists in practice probably always go with what looks
like the best number, and that makes sense, but I look at the composite
picture.
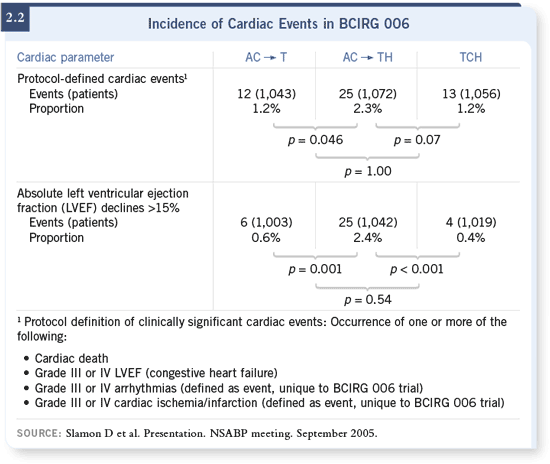
We presented the data on cardiac safety from 006 (2.2), and the results are
profoundly different from TCH, so it’ll all depend on the weight of the
efficacy data when we have sufficient numbers and how that stands up against
the safety data.
If you look at left ventricular dysfunction progressively over time, both anthracycline-
containing arms do worse. The information has been around a while
that nontrastuzumab/anthracycline regimens do make an impact, and we
have learned from the adjuvant trials with trastuzumab that we make an even
bigger impact than we previously thought.
We thought we were out of the woods with the anthracycline doses we’re
using, and now we’ve found, as Chuck Geyer pointed out that the incidence
is much higher than we thought, even for the standard arm, and this
concerns me.
That has to be weighed against efficacy, and if the efficacy is strongly and
statistically significantly different, then I think that has to be taken into
consideration when you treat patients. We don’t have data sufficient to speak
to that at this point, but we will soon and when that data is available, it needs
to be made public.
 Track 5 Track 5
 DR LOVE: How would you treat a woman in her fifties with nodepo-sitive,
HER2-positive disease? DR LOVE: How would you treat a woman in her fifties with nodepo-sitive,
HER2-positive disease? |
 DR SLAMON: We use TCH and will continue to do so until we see that it is
inferior, and the safety profile doesn’t make up for that inferiority. DR SLAMON: We use TCH and will continue to do so until we see that it is
inferior, and the safety profile doesn’t make up for that inferiority.
 DR LOVE: Can we anticipate that the relative risk reduction of trastuzumab chemotherapy
regimens will extend to patients who have node-negative
disease, particularly with smaller tumors? DR LOVE: Can we anticipate that the relative risk reduction of trastuzumab chemotherapy
regimens will extend to patients who have node-negative
disease, particularly with smaller tumors?
 DR SLAMON: I would think so. Based on the biology of the disease, we know
that when the HER2 alteration is present, it’s a more aggressive disease. Now
if you have a patient who has a smaller tumor, it has to be weighed against the
likelihood of cure from the initial therapy — surgery and radiation therapy. DR SLAMON: I would think so. Based on the biology of the disease, we know
that when the HER2 alteration is present, it’s a more aggressive disease. Now
if you have a patient who has a smaller tumor, it has to be weighed against the
likelihood of cure from the initial therapy — surgery and radiation therapy.
We also know there’s a subpopulation of patients who are HER2-positive, who
aren’t cured, even if they have very small tumors. Treating with trastuzumab
in the metastatic versus the adjuvant setting clearly isn’t as advantageous.
My feeling is that we’re dealing with a relatively benign agent in trastuzumab,
when used correctly. It’s an enormously expensive drug, and that has to be put into context, but we have the two goalposts set. We know the drug is effective
in the adjuvant setting and in metastatic disease. Between those two goalposts,
I think it can be very effective.
 Track 7 Track 7
 DR LOVE: What are your thoughts about the use of adjuvant trastuzumab
monotherapy without chemotherapy in patients with comorbidities or of
advanced age? DR LOVE: What are your thoughts about the use of adjuvant trastuzumab
monotherapy without chemotherapy in patients with comorbidities or of
advanced age? |
 DR SLAMON: The orthodox answer is “no” — not outside the context of a
clinical trial. My answer, and take it as my personal answer, is that the drug
is effective, and if you’re dealing with a patient not on a clinical trial, it’s an
individual sitting in front of you and the art of medicine still applies. If they’ve
got significant comorbid disease, I would not withhold an effective therapy
(Vogel 2002) because they don’t meet some protocol. I would administer
single-agent trastuzumab. DR SLAMON: The orthodox answer is “no” — not outside the context of a
clinical trial. My answer, and take it as my personal answer, is that the drug
is effective, and if you’re dealing with a patient not on a clinical trial, it’s an
individual sitting in front of you and the art of medicine still applies. If they’ve
got significant comorbid disease, I would not withhold an effective therapy
(Vogel 2002) because they don’t meet some protocol. I would administer
single-agent trastuzumab.
 Track 8 Track 8
 DR LOVE: Most physicians seem to be using adjuvant trastuzumab for one
year. Is that what you’re doing? DR LOVE: Most physicians seem to be using adjuvant trastuzumab for one
year. Is that what you’re doing? |
 DR SLAMON: Yes, but there are no clinical data to tell us how long to administer
the drug. The one-year duration came from sort of an empiric extension
of preclinical data, and my sense, based on those data, is that the optimal
duration will be somewhere between six and 12 months. DR SLAMON: Yes, but there are no clinical data to tell us how long to administer
the drug. The one-year duration came from sort of an empiric extension
of preclinical data, and my sense, based on those data, is that the optimal
duration will be somewhere between six and 12 months.
Also, based on that same preclinical data, I don’t think there’ll be a big difference
between one and two years. One good thing about the HERA trial is
that it’s asking that question so we’ll get that information (2.3).
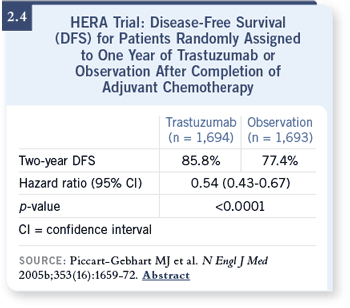
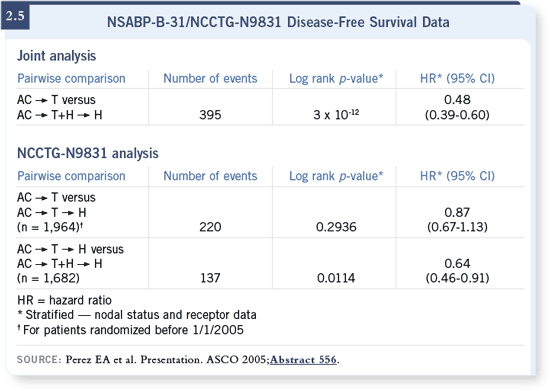
 DR LOVE: It’s a little difficult to interpret the data from the HERA trial as
opposed to the NCCTG trial with regard to sequential versus concurrent
therapy. The HERA study showed approximately a 50 percent risk reduction
(Piccart-Gebhart 2005b; [2.4]), as was seen in the combined analysis (Romond
2005b). However, in the NCCTG trial there’s a 13 percent nonstatistically
significant reduction in the sequential arm (Perez 2005; [2.5]). How do you
interpret those data? DR LOVE: It’s a little difficult to interpret the data from the HERA trial as
opposed to the NCCTG trial with regard to sequential versus concurrent
therapy. The HERA study showed approximately a 50 percent risk reduction
(Piccart-Gebhart 2005b; [2.4]), as was seen in the combined analysis (Romond
2005b). However, in the NCCTG trial there’s a 13 percent nonstatistically
significant reduction in the sequential arm (Perez 2005; [2.5]). How do you
interpret those data?
 DR SLAMON: I think it’s far too early to make a call, and I think that’s both
the safe as well as the right answer. The HERA investigators have openly
conceded the point that their data are more immature, vis-à-vis trastuzumab
therapy, given the fact that they had an even longer period before the patients
were randomized to trastuzumab, so their follow-up time is short. DR SLAMON: I think it’s far too early to make a call, and I think that’s both
the safe as well as the right answer. The HERA investigators have openly
conceded the point that their data are more immature, vis-à-vis trastuzumab
therapy, given the fact that they had an even longer period before the patients
were randomized to trastuzumab, so their follow-up time is short.
 Now they make up for that,
in part, by large numbers,
because they enrolled a lot
of patients on the trial. But
I think we need one more
look at those data in terms of
an update before we’re really
able to say that they’re seeing
the same kind of impact that
we are seeing with the two
cooperative group trials in
the United States.
Now they make up for that,
in part, by large numbers,
because they enrolled a lot
of patients on the trial. But
I think we need one more
look at those data in terms of
an update before we’re really
able to say that they’re seeing
the same kind of impact that
we are seeing with the two
cooperative group trials in
the United States.
 DR LOVE: The sequential
versus concurrent arms in
the NCCTG trial don’t have
very many events at this
point, so could it be that that’s the trial that’s misleading? DR LOVE: The sequential
versus concurrent arms in
the NCCTG trial don’t have
very many events at this
point, so could it be that that’s the trial that’s misleading?
 DR SLAMON: It’s true that the study doesn’t have many events, but it has
longer follow-up. DR SLAMON: It’s true that the study doesn’t have many events, but it has
longer follow-up.
 Track 9 Track 9
 DR LOVE: What about the issue of delayed trastuzumab? Should the
patient with a HER2-positive tumor who’s now six months, 12 months,
or a couple of years after initial diagnosis be offered trastuzumab? DR LOVE: What about the issue of delayed trastuzumab? Should the
patient with a HER2-positive tumor who’s now six months, 12 months,
or a couple of years after initial diagnosis be offered trastuzumab? |
There’s the orthodox answer, and there’s what I think is the biologic answer.
The orthodox answer is, there are no data that address whether giving it out
further will be beneficial, and there’s no trial with any data to speak to that.
The biologic answer is very similar to what I said earlier. You know trastuzumab
works in the adjuvant and in the metastatic setting. The patient you’re
talking about sits between those two goalposts. If she has a disease that has
escaped her primary site and it’s a HER2-positive tumor, the likelihood of
her receiving benefit from trastuzumab is there. So my sense is, biologically, it
should be beneficial.
 DR LOVE: In the clinical use of delayed aromatase inhibitor therapy, there’s
the mindset that the decision should be determined by the patient’s risk at
that point, and assuming therapy would significantly decrease that risk. Do
you believe that approach makes sense in terms of initiating delayed adjuvant
trastuzumab? DR LOVE: In the clinical use of delayed aromatase inhibitor therapy, there’s
the mindset that the decision should be determined by the patient’s risk at
that point, and assuming therapy would significantly decrease that risk. Do
you believe that approach makes sense in terms of initiating delayed adjuvant
trastuzumab?
 DR SLAMON: I think that’s absolutely the way to go, but I don’t think we
have sufficient parameters or parametrics to measure that at this time. DR SLAMON: I think that’s absolutely the way to go, but I don’t think we
have sufficient parameters or parametrics to measure that at this time.
 Track 11 Track 11
 DR LOVE: How should patients who received prior adjuvant trastuzumab
be managed at relapse? DR LOVE: How should patients who received prior adjuvant trastuzumab
be managed at relapse? |
 DR SLAMON: The thinking on treatment after progression following adjuvant
trastuzumab falls into two camps: Whether it makes sense to try something
entirely different or to continue trastuzumab and add something new. The
data we’re seeing, based on preclinical information, would indicate that the
latter makes sense. However, there are no clinical data to speak to that, and it’s going to be almost impossible to do a clinical trial to address it because of the
half-life of the antibody. DR SLAMON: The thinking on treatment after progression following adjuvant
trastuzumab falls into two camps: Whether it makes sense to try something
entirely different or to continue trastuzumab and add something new. The
data we’re seeing, based on preclinical information, would indicate that the
latter makes sense. However, there are no clinical data to speak to that, and it’s going to be almost impossible to do a clinical trial to address it because of the
half-life of the antibody.
We know that trastuzumab sticks around for a long period of time, so you’d
be hard pressed to find a patient or an oncologist willing to wait until all
the trastuzumab washes out before starting therapy in the face of progressive
disease.
So by definition, if you switch to a different chemotherapy and you randomize
to no continuation of trastuzumab versus continuation of trastuzumab, at week
three when you add that chemotherapy, you’re still doing a combination study.
That’s what makes it impossible to ask the question.
 DR LOVE: Then what is your conclusion on this issue? How do you treat these
patients? DR LOVE: Then what is your conclusion on this issue? How do you treat these
patients?
 DR SLAMON: The only conclusion I have is that if you’ve had a response to
trastuzumab to begin with, I would continue trastuzumab therapy. The only
place where I don’t do that — based on no data, just sort of my gut feeling and
what we know about the biology — is when the patient progresses within a
few months of having stopped their chemotherapy, I’m less likely to continue
trastuzumab. However, if the patient is slowly progressing through the trastuzumab
therapy, there still may be an effect of the antibody, and we can add
something different to it. DR SLAMON: The only conclusion I have is that if you’ve had a response to
trastuzumab to begin with, I would continue trastuzumab therapy. The only
place where I don’t do that — based on no data, just sort of my gut feeling and
what we know about the biology — is when the patient progresses within a
few months of having stopped their chemotherapy, I’m less likely to continue
trastuzumab. However, if the patient is slowly progressing through the trastuzumab
therapy, there still may be an effect of the antibody, and we can add
something different to it.
 Track 15 Track 15
 DR LOVE: Can you comment on the work that your group has done
evaluating bevacizumab plus trastuzumab and whether you see that
moving into the adjuvant setting? DR LOVE: Can you comment on the work that your group has done
evaluating bevacizumab plus trastuzumab and whether you see that
moving into the adjuvant setting? |
 DR SLAMON: I certainly hope it moves into the adjuvant setting. I think
there’s more than enough very strong, compelling biologic and preclinical data
indicating that this combination is rational. DR SLAMON: I certainly hope it moves into the adjuvant setting. I think
there’s more than enough very strong, compelling biologic and preclinical data
indicating that this combination is rational.
There are now early clinical data, with very small numbers, both in the Phase
I and II settings, which indicate that this is an active regimen, so I think it’s
ready to be moved into a larger trial. My sense is that in short order it should
be ready to be moved into the adjuvant setting. We know how trastuzumab
performs in the HER2-positive population, and the efficacy of bevacizumab
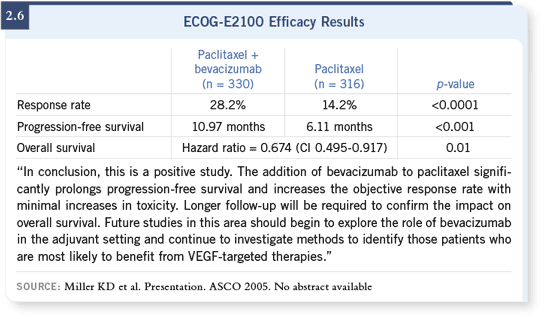
was demonstrated in the ECOG-E2100 study (Miller 2005 [2.6]).
We now know — based not only on preclinical but clinical data — that the
HER2 population has a higher VEGF level. All of the dots connect. Now
it’s a matter of showing that the combination is safe and showing some clear
efficacy data that will allow us to think about launching a larger study.
 DR LOVE: Can you talk more specifically about the Phase I and II data on the
trastuzumab/bevacizumab combination? DR LOVE: Can you talk more specifically about the Phase I and II data on the
trastuzumab/bevacizumab combination?
 DR SLAMON: The Phase I data were with nine patients, three in one of three
groups (Pegram 2004). The study evaluated three different doses of bevacizumab,
because we didn’t know the right dose when we launched it, along
with standard-dose trastuzumab. DR SLAMON: The Phase I data were with nine patients, three in one of three
groups (Pegram 2004). The study evaluated three different doses of bevacizumab,
because we didn’t know the right dose when we launched it, along
with standard-dose trastuzumab.
We saw responses in all three groups. Nine patients is a very small number,
but the responses were pretty compelling in a first-line metastatic group. In
addition, the third cohort was actually allowed beyond first-line treatment.
What we found was, in that total group of nine, there was one complete
response, four partial responses, two stable diseases for greater than 11 months,
and two patients who progressed. That was pretty exciting data for a small
group, but you have to take that with a grain of salt because it’s only with
nine patients.
We’ve now expanded it into a Phase II study, looking at the two biologics
together in first-line metastatic disease. What we’re seeing up to this point is
very similar to what we saw in the Phase I trial. We have 20 patients now in
the Phase II, so a total experience with about 29 patients, and we’re seeing the
same kinds of response rates. We’re very encouraged by the data. It needs to
mature further, but I think it’s a rational regimen, and I think it’ll make its
way to the clinic in terms of a big clinical study.
 DR LOVE: Do you think this combination is ready to go into the adjuvant
setting now? DR LOVE: Do you think this combination is ready to go into the adjuvant
setting now?
 DR SLAMON: I think it will go to the adjuvant setting. It’s ready, but I tend to
be a little more aggressive about looking at these things, maybe, than others.
However, I would wait until we finish this Phase II study, which is supposed
to accrue 50 patients and look at the response rate and duration in those
patients. We’ll know that relatively soon. DR SLAMON: I think it will go to the adjuvant setting. It’s ready, but I tend to
be a little more aggressive about looking at these things, maybe, than others.
However, I would wait until we finish this Phase II study, which is supposed
to accrue 50 patients and look at the response rate and duration in those
patients. We’ll know that relatively soon.
 DR LOVE: Knowing the dangers of interpreting anecdotal cases or small
series, other than the numbers in terms of response rates, is the magnitude
of responses seen in the Phase I and II trials greater than you would have
expected with trastuzumab alone? DR LOVE: Knowing the dangers of interpreting anecdotal cases or small
series, other than the numbers in terms of response rates, is the magnitude
of responses seen in the Phase I and II trials greater than you would have
expected with trastuzumab alone?
 DR SLAMON: Absolutely. I would not have expected it with trastuzumab
alone or bevacizumab alone. If we were only basing it on thinking these might
work well together versus the fact that the preclinical gene array data directed
us, then I’d be a little more concerned, but given the fact that there are strong
preclinical data, and now clinical data, I’m comfortable with it. DR SLAMON: Absolutely. I would not have expected it with trastuzumab
alone or bevacizumab alone. If we were only basing it on thinking these might
work well together versus the fact that the preclinical gene array data directed
us, then I’d be a little more concerned, but given the fact that there are strong
preclinical data, and now clinical data, I’m comfortable with it.
There’s an enormous amount of preclinical data indicating that the pathways
of the two agents are linked, which is compelling and reproducible. You can
show that HER2-positive tumors have VEGF levels that are much higher than
the general breast cancer population by gene array analysis.
 DR LOVE: When you say the VEGF levels are high, is that a direct stimulant
in terms of tumor growth? DR LOVE: When you say the VEGF levels are high, is that a direct stimulant
in terms of tumor growth?
 DR SLAMON: Yes. We believe it’s a direct stimulant. We can’t test that in
the clinic, but we can test it preclinically. In the clinic, we know the HER2-
positive tumors — age-matched, stage-matched controlled — are more
metastatic and grow more rapidly. Now there’s direct growth stimulation
of the HER2 pathway itself, and there’s all the ancillary things you need to
support faster and more aggressive tumor growth, not the least of which is
neoangiogenesis, so it all makes sense. DR SLAMON: Yes. We believe it’s a direct stimulant. We can’t test that in
the clinic, but we can test it preclinically. In the clinic, we know the HER2-
positive tumors — age-matched, stage-matched controlled — are more
metastatic and grow more rapidly. Now there’s direct growth stimulation
of the HER2 pathway itself, and there’s all the ancillary things you need to
support faster and more aggressive tumor growth, not the least of which is
neoangiogenesis, so it all makes sense.
 DR LOVE: Do you believe bevacizumab potentiates chemotherapy by
improving delivery and that’s why it would work well with trastuzumab, or
do you believe that, at least in HER2-positive breast cancer, there’s something
else going on with that combination? DR LOVE: Do you believe bevacizumab potentiates chemotherapy by
improving delivery and that’s why it would work well with trastuzumab, or
do you believe that, at least in HER2-positive breast cancer, there’s something
else going on with that combination?
 DR SLAMON: I’m more in the camp of the latter, thinking there’s something
else going on. There are interesting data, including some data from clinical
material, indicating that there may be this gradient phenomenon in terms of
better penetration of chemotherapy. However, when you look at the doses
achieved within the tissue, even when this is present, it’s well above the IC50s. DR SLAMON: I’m more in the camp of the latter, thinking there’s something
else going on. There are interesting data, including some data from clinical
material, indicating that there may be this gradient phenomenon in terms of
better penetration of chemotherapy. However, when you look at the doses
achieved within the tissue, even when this is present, it’s well above the IC50s.
The question is: Are higher doses achieved due to increased oncotic pressure,
which is thought to be one of the mechanisms of VEGF? It’s clear that the
VEGF antibody will tie up circulating VEGF and that VEGF is one component
needed for neoangiogenesis for a number of different kinds of tumors, so
I think there is a lot of room for other mechanisms of how that would work.
 DR LOVE: Do you believe that changing the oncotic pressure or profusion
theoretically should be beneficial in terms of trastuzumab delivery? DR LOVE: Do you believe that changing the oncotic pressure or profusion
theoretically should be beneficial in terms of trastuzumab delivery?
 DR SLAMON: Theoretically, but I would want to see supporting data that are
reproducible. DR SLAMON: Theoretically, but I would want to see supporting data that are
reproducible.
Select publications
|

