|

| Tracks 1-14 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Evolution of the HERA trial design |
| Track 3 |
Efficacy results of the HERA trial |
| Track 4 |
Cardiac toxicity observed in the
HERA trial |
| Track 5 |
Concurrent versus sequential
adjuvant trastuzumab |
| Track 6 |
Implications of BCIRG 006
clinical trial results |
| Track 7 |
Incorporating adjuvant trastuzumab
into clinical practice |
| Track 8 |
Clinical use of trastuzumab with
hormonal therapy |
|
| Track 9 |
Delayed adjuvant trastuzumab in
patients with high-risk disease |
| Track 10 |
Combining adjuvant trastuzumab
with dose-dense chemotherapy |
| Track 11 |
Future research strategies in
patients with HER2-positive
disease |
| Track 12 |
Clinical trials of trastuzumab and
bevacizumab |
| Track 13 |
Neoadjuvant trastuzumab |
| Track 14 |
Reflections on progress in
targeted therapy in oncology |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Can you describe the background of the HERA study? DR LOVE: Can you describe the background of the HERA study? |
 DR LEYLAND-JONES: The design of the HERA study came about in a very
pragmatic way. A group of us met on a Saturday in Frankfurt and spent a
number of hours trying to come up with a common regimen. It became clear
that it was impossible to agree upon one combined regimen. DR LEYLAND-JONES: The design of the HERA study came about in a very
pragmatic way. A group of us met on a Saturday in Frankfurt and spent a
number of hours trying to come up with a common regimen. It became clear
that it was impossible to agree upon one combined regimen.
The following morning, we simply drew the current trial design, in which
we decided to include any prior chemotherapy regimen within reason, and
mandated that eligible patients must have completed at least four cycles
(Piccart-Gebhart 2005). We also decided to adopt the three-weekly trastuzumab
regimen, which had been tested extensively worldwide.
Another factor that drove the trial design was the fact that it takes 18 weeks,
either on the weekly or the three-weekly schedule, to reach steady-state levels
of trastuzumab. So basically, with one year of therapy, you’ve devoted over a third of the treatment time in achieving steady state. We decided to include
a two-year arm also. That was supportive of the data that came from Rich
Pietras and Dennis Slamon’s group, which indicated that you need continuous
attenuation of HER2 signaling to derive the most benefit (Pietras 1998).
So the four characteristics of the initial trial design were: Patients had to
receive adjuvant chemotherapy; trastuzumab was administered following
the completion of all chemotherapy, radiation and surgery; the three-weekly
trastuzumab regimen was adopted; and a three-arm design of observation
versus one year of trastuzumab versus two years of trastuzumab was developed.
 DR LOVE: What were the determinants of patient eligibility? DR LOVE: What were the determinants of patient eligibility?
 DR LEYLAND-JONES: There were many discussions about how patients with
node-negative disease fared if they had poor pathology. So we decided to
include anyone with a tumor size of greater than one centimeter, including
patients with node-negative disease. We did not include patients with inflammatory
breast cancer. One third of patients enrolled in the trial had nodenegative
disease. DR LEYLAND-JONES: There were many discussions about how patients with
node-negative disease fared if they had poor pathology. So we decided to
include anyone with a tumor size of greater than one centimeter, including
patients with node-negative disease. We did not include patients with inflammatory
breast cancer. One third of patients enrolled in the trial had nodenegative
disease.
 Track 3 Track 3
 DR LOVE: Can you talk about the results that were observed? DR LOVE: Can you talk about the results that were observed? |
 DR LEYLAND-JONES: The findings were striking. The three-arm trial design
mandated the accrual of more than 4,500 patients. In practice, accrual reached
5,070 women. The first interim analysis was planned at 475 events. That
analysis showed a hazard ratio of 0.54 (p < 0.0001) for disease-free survival,
which was remarkable after only one year of median follow-up (Piccart-Gebhart 2005). DR LEYLAND-JONES: The findings were striking. The three-arm trial design
mandated the accrual of more than 4,500 patients. In practice, accrual reached
5,070 women. The first interim analysis was planned at 475 events. That
analysis showed a hazard ratio of 0.54 (p < 0.0001) for disease-free survival,
which was remarkable after only one year of median follow-up (Piccart-Gebhart 2005).
 DR LOVE: What about the survival data? DR LOVE: What about the survival data?
 DR LEYLAND-JONES: Survival was not yet statistically significant after one
year of median follow-up. That will be pursued, and additional data may be
presented at the 2005 San Antonio Breast Cancer Symposium. DR LEYLAND-JONES: Survival was not yet statistically significant after one
year of median follow-up. That will be pursued, and additional data may be
presented at the 2005 San Antonio Breast Cancer Symposium.
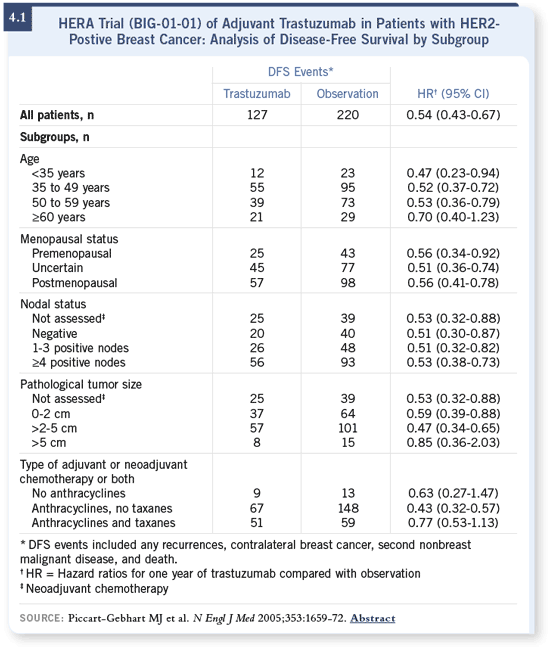
 DR LOVE: When you look at different patient subsets, particularly according
to status, age and other factors, what did you see in terms of relative risk
reduction? DR LOVE: When you look at different patient subsets, particularly according
to status, age and other factors, what did you see in terms of relative risk
reduction?
 DR LEYLAND-JONES: As has been common across most of the trials, the data
have not shown any huge differences between the subsets in general (4.1).
However, the HERA study is the only trial in which one third of the patients
had node-negative disease, and in this respect, the data are utterly striking. DR LEYLAND-JONES: As has been common across most of the trials, the data
have not shown any huge differences between the subsets in general (4.1).
However, the HERA study is the only trial in which one third of the patients
had node-negative disease, and in this respect, the data are utterly striking.
Everything falls the same way, with exactly the same hazard ratio, whether it’s
a subgroup with negative nodal status, one to three positive nodes or four or
more positive nodes. This gives us enormous confidence to treat node-negative
disease with the same regimens that we utilize to treat node-positive disease.
 This is all a credit to the work of Dennis Slamon (Slamon 1987), who recognized
that the expression of HER2 was critical to tumor biology and that this
is a beautifully targeted therapy. In Melody Cobleigh’s study of trastuzumab
as a second- or third-line single agent in patients with HER2-positive disease,
there was an objective response rate of approximately 15 percent (Cobleigh
1999). That objective response increased to almost 35 percent in Chuck
Vogel’s study, when it went into use as front-line therapy (Vogel 2002).
This is all a credit to the work of Dennis Slamon (Slamon 1987), who recognized
that the expression of HER2 was critical to tumor biology and that this
is a beautifully targeted therapy. In Melody Cobleigh’s study of trastuzumab
as a second- or third-line single agent in patients with HER2-positive disease,
there was an objective response rate of approximately 15 percent (Cobleigh
1999). That objective response increased to almost 35 percent in Chuck
Vogel’s study, when it went into use as front-line therapy (Vogel 2002).
We’ve seen huge incremental gains with docetaxel/trastuzumab combinations
and paclitaxel/carboplatin/trastuzumab combinations as front-line treatments.
In many ways, we expected trastuzumab to be associated with increases in efficacy during the adjuvant studies; however, I don’t believe any of us
thought it would be this dramatic.
 DR LOVE: What did you see when you looked at the different chemotherapeutic
regimens that patients received in the HERA trial? Were you able to
tease anything out of that analysis? DR LOVE: What did you see when you looked at the different chemotherapeutic
regimens that patients received in the HERA trial? Were you able to
tease anything out of that analysis?
 DR LEYLAND-JONES: Around two thirds of the patients were treated with a
straight anthracycline regimen: FAC, FEC or AC. Approximately six percent
were treated with a nonanthracycline/nontaxane-containing regimen such as
CMF. Around 25 percent were treated with an anthracycline/taxane. DR LEYLAND-JONES: Around two thirds of the patients were treated with a
straight anthracycline regimen: FAC, FEC or AC. Approximately six percent
were treated with a nonanthracycline/nontaxane-containing regimen such as
CMF. Around 25 percent were treated with an anthracycline/taxane.
So the anthracycline/taxane group appeared to do worse, although the confidence
intervals are fairly wide because it’s a smaller number (4.1). Patients
treated with an anthracycline/taxane regimen tend to be a group with a worse
prognosis. They tend to have large numbers of positive nodes, larger tumors
and worse pathologies.
After essentially any adjuvant chemotherapy regimen with trastuzumab given
sequentially, the hazard ratio is 0.54, and there was a significantly decreased
incidence of distant metastases. So it shows the power of administering
trastuzumab sequentially.
 Track 4 Track 4
 DR LOVE: Can you discuss cardiac toxicity in the HERA trial? DR LOVE: Can you discuss cardiac toxicity in the HERA trial? |
 DR LEYLAND-JONES: That is one of the key features of this trial. The entry
criteria included completion of chemotherapy, surgery and radiation therapy
prior to trastuzumab. Therefore, the randomization took place with trastuzumab
starting either six weeks after completion of the radiation or surgery or
seven weeks after the last cycle of chemotherapy. DR LEYLAND-JONES: That is one of the key features of this trial. The entry
criteria included completion of chemotherapy, surgery and radiation therapy
prior to trastuzumab. Therefore, the randomization took place with trastuzumab
starting either six weeks after completion of the radiation or surgery or
seven weeks after the last cycle of chemotherapy.
This is a very clear separation, and what this resulted in was a difference in
Grade III/IV cardiac toxicity, which was 0.54 percent in the trastuzumab
group versus zero percent in the observation group (4.2).
We have to remember that the denominator is approximately 1,700 patients.
In hard numbers, Grade III/IV CHF occurred in nine patients versus zero. In
contrast, the Intergroup data reported CHF in 31 patients who received trastuzumab
and in four patients from the control group (Romond 2005). So there
was considerably less cardiac toxicity in the HERA trial if you’re looking at
cross-trial data.
The Intergroup study had a median follow-up of two years, whereas follow-up
in HERA was one year. There are differences between the trials, of course.
The LVEF cutoff criterion in the HERA study was 55 percent as opposed
to 50 percent in the Intergroup trial. Having said that, at least looking at the
HERA data, per se, there was a very low risk of cardiac toxicity.
 DR LOVE: What is your current adjuvant treatment approach for patients with
HER2-positive breast cancer? DR LOVE: What is your current adjuvant treatment approach for patients with
HER2-positive breast cancer?
 DR LEYLAND-JONES: For our younger, fit patients, we use the current Intergroup/
NSABP method: AC DR LEYLAND-JONES: For our younger, fit patients, we use the current Intergroup/
NSABP method: AC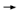 TH, exactly as in NSABP-B-31. The NSABP
and Intergroup trials showed that older patients or those who started post-AC
with an LVEF in the 50 to 54 range were at a much higher risk for cardiac
toxicity. Therefore, we tend to use a HERA type of regimen in the older
patients or in patients who have a lower LVEF. TH, exactly as in NSABP-B-31. The NSABP
and Intergroup trials showed that older patients or those who started post-AC
with an LVEF in the 50 to 54 range were at a much higher risk for cardiac
toxicity. Therefore, we tend to use a HERA type of regimen in the older
patients or in patients who have a lower LVEF.
 Track 5 Track 5
 DR LOVE: What are your thoughts about the NCCTG analysis, evaluating
concurrent versus sequential trastuzumab therapy? The sequential
treatment was similar to the HERA approach, yet there the NCCTG
didn’t see a statistically significant drop in relapse rate. You saw a 50
percent drop. DR LOVE: What are your thoughts about the NCCTG analysis, evaluating
concurrent versus sequential trastuzumab therapy? The sequential
treatment was similar to the HERA approach, yet there the NCCTG
didn’t see a statistically significant drop in relapse rate. You saw a 50
percent drop.
|
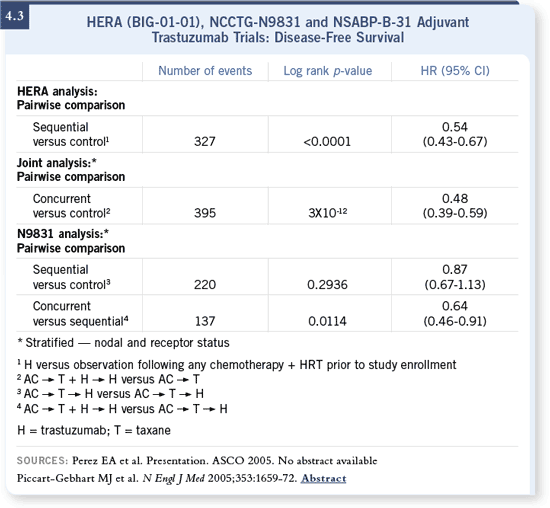
 DR LEYLAND-JONES: It’s an excellent question. All I can say is, the results are
early, and the median follow-up is relatively short. If you look at the HERA data, the hazard ratio is 0.54 for disease-free survival. The cardiac toxicity rate
is low, and the efficacy and safety results are based on more than 1,600 patients
per arm. DR LEYLAND-JONES: It’s an excellent question. All I can say is, the results are
early, and the median follow-up is relatively short. If you look at the HERA data, the hazard ratio is 0.54 for disease-free survival. The cardiac toxicity rate
is low, and the efficacy and safety results are based on more than 1,600 patients
per arm.
In the NCCTG study, the number of events in the concurrent versus sequential
analysis is small — about 130 events (4.3). The complete number of events
expected are 530, so they are only about a quarter of the way through the
data. The idea that synergy between agents exists may well pan out in the
end, and giving these drugs concurrently is perhaps better than giving them
sequentially.
 Track 8 Track 8
 DR LOVE: What was seen in the HERA trial in patients with ER-positive
and HER2-positive tumors? DR LOVE: What was seen in the HERA trial in patients with ER-positive
and HER2-positive tumors? |
 DR LEYLAND-JONES: The ER-positive population was half the population.
Equal benefit from trastuzumab was seen in the ER-positive population.
Again, I believe this is another indicator of the importance of HER2 biology. DR LEYLAND-JONES: The ER-positive population was half the population.
Equal benefit from trastuzumab was seen in the ER-positive population.
Again, I believe this is another indicator of the importance of HER2 biology.
 DR LOVE: In the clinical setting, how do you approach the use of hormonal
therapy with these patients? In the trials, patients received hormonal therapy
together with trastuzumab after chemotherapy was completed. Is that your
general approach? DR LOVE: In the clinical setting, how do you approach the use of hormonal
therapy with these patients? In the trials, patients received hormonal therapy
together with trastuzumab after chemotherapy was completed. Is that your
general approach?
 DR LEYLAND-JONES: We utilize chemotherapy and trastuzumab followed by
an aromatase inhibitor in patients who have HER2-positive and ER-positive
disease. The original protocol for HERA included only tamoxifen. It was
subsequently modified to include the aromatase inhibitors. As shown in the
2004 San Antonio presentation, many patients with ER-positive, HER2-
positive disease have a downregulation of the PR, and the aromatase inhibitors
are better in this treatment group (Dowsett 2005). DR LEYLAND-JONES: We utilize chemotherapy and trastuzumab followed by
an aromatase inhibitor in patients who have HER2-positive and ER-positive
disease. The original protocol for HERA included only tamoxifen. It was
subsequently modified to include the aromatase inhibitors. As shown in the
2004 San Antonio presentation, many patients with ER-positive, HER2-
positive disease have a downregulation of the PR, and the aromatase inhibitors
are better in this treatment group (Dowsett 2005).
 DR LOVE: So you start the aromatase inhibitor after the chemotherapy? DR LOVE: So you start the aromatase inhibitor after the chemotherapy?
 DR LEYLAND-JONES: Yes. DR LEYLAND-JONES: Yes.
 DR LOVE: In the metastatic setting, do you utilize a combination of hormonal
therapy and trastuzumab? DR LOVE: In the metastatic setting, do you utilize a combination of hormonal
therapy and trastuzumab?
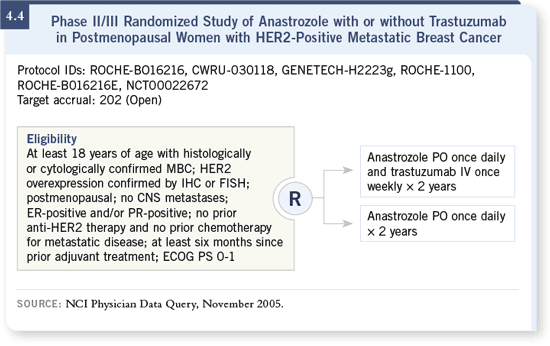
 DR LEYLAND-JONES: We are participating in a trial of anastrozole with or
without trastuzumab (4.4). We already have first-hand experience with that
combination; however, the data are not yet available for this trial. The natural
biology drives the use of this combination, so we utilize trastuzumab and
aromatase inhibitors in select patients in the clinical setting. DR LEYLAND-JONES: We are participating in a trial of anastrozole with or
without trastuzumab (4.4). We already have first-hand experience with that
combination; however, the data are not yet available for this trial. The natural
biology drives the use of this combination, so we utilize trastuzumab and
aromatase inhibitors in select patients in the clinical setting.
 Track 9 Track 9
 DR LOVE: The patient with multiple positive nodes — five to 10 positive
nodes — who is a year or two out from chemotherapy is still at substantial
risk for subsequent recurrence. Do you think trastuzumab should be
discussed with these patients? DR LOVE: The patient with multiple positive nodes — five to 10 positive
nodes — who is a year or two out from chemotherapy is still at substantial
risk for subsequent recurrence. Do you think trastuzumab should be
discussed with these patients? |
 DR LEYLAND-JONES: This is an agonizing situation. The evidence is the
evidence, and national advisory committees everywhere use it. The general
advice is to restrict the use of adjuvant trastuzumab to within six months
of completing chemotherapy. One or two guidelines are taking it out to a
year. At our center in Canada, however, we are restricted by the data, which
support utilizing trastuzumab within six months of completing chemotherapy. DR LEYLAND-JONES: This is an agonizing situation. The evidence is the
evidence, and national advisory committees everywhere use it. The general
advice is to restrict the use of adjuvant trastuzumab to within six months
of completing chemotherapy. One or two guidelines are taking it out to a
year. At our center in Canada, however, we are restricted by the data, which
support utilizing trastuzumab within six months of completing chemotherapy.
 Track 10 Track 10
 DR LOVE: What about dose-dense chemotherapy and trastuzumab? DR LOVE: What about dose-dense chemotherapy and trastuzumab? |
 DR LEYLAND-JONES: We’ve treated patients with that regimen. A number
of people question the best way of incorporating the trastuzumab. We do not
have the appropriate pharmacokinetic data on this, but a number of doctors
are administering a 4 mg/kg two-weekly regimen. DR LEYLAND-JONES: We’ve treated patients with that regimen. A number
of people question the best way of incorporating the trastuzumab. We do not
have the appropriate pharmacokinetic data on this, but a number of doctors
are administering a 4 mg/kg two-weekly regimen.
There is some evidence that using loading doses can help. Instead of giving
the 2 mg/kg weekly dose, it makes sense to combine it with the dose-dense
chemotherapy regimen using a 4 mg/kg two-weekly regimen, which makes it
easier for the patient during the time of transition from AC to trastuzumab. In
a clinical setting, it is natural that physicians would adopt trastuzumab into a
dose-dense kind of regimen.
 Track 13 Track 13
Neoadjuvant trastuzumab
 DR LOVE: What were your thoughts about the data from Aman Buzdar
evaluating neoadjuvant trastuzumab/chemotherapy? DR LOVE: What were your thoughts about the data from Aman Buzdar
evaluating neoadjuvant trastuzumab/chemotherapy? |
 DR LEYLAND-JONES: This study had one of the most dramatic complete
response rates ever seen in the neoadjuvant setting. The trial was discontinued
after accruing 34 patients because it showed a huge difference between
the two arms (Buzdar 2005). This causes a dilemma, because you can show
patients a 67 percent pCR rate with the combined neoadjuvant regimen;
however, the regulatory authorities look at this and say, “Well, you have 18
patients in the one arm and 16 in the other. Show us something that is more
significant clinically.” DR LEYLAND-JONES: This study had one of the most dramatic complete
response rates ever seen in the neoadjuvant setting. The trial was discontinued
after accruing 34 patients because it showed a huge difference between
the two arms (Buzdar 2005). This causes a dilemma, because you can show
patients a 67 percent pCR rate with the combined neoadjuvant regimen;
however, the regulatory authorities look at this and say, “Well, you have 18
patients in the one arm and 16 in the other. Show us something that is more
significant clinically.”
Unfortunately, because the data are so compelling, I’m not sure whether we
are going to have a large neoadjuvant trial. At the moment, the vast majority of practicing oncologists are using trastuzumab in the neoadjuvant setting. The
appropriate regimen is not well demonstrated, and we do not have a large trial
confirming the benefit of trastuzumab.
Select publications
|

