
Select Excerpts from the Discussion
CD 2, Track 14
 DR LOVE: Cliff, can you comment on the updated results from the
HERA trial that were presented at ASCO 2006 by Ian Smith?
DR LOVE: Cliff, can you comment on the updated results from the
HERA trial that were presented at ASCO 2006 by Ian Smith?
 DR HUDIS: I was struck that the hazard for recurrence actually “blipped”
upward after the year of trastuzumab ended (Smith 2006). I believe that leaves
the door wide open that prolonged treatment may be better than stopping
after one year.
DR HUDIS: I was struck that the hazard for recurrence actually “blipped”
upward after the year of trastuzumab ended (Smith 2006). I believe that leaves
the door wide open that prolonged treatment may be better than stopping
after one year.
Many were excited because of the FinHer data, which demonstrated that nine
weeks of trastuzumab significantly lowered the risk of recurrence or death
( Joensuu 2006). I’m not disputing that result. However, we don’t have the data
for two years versus one year of trastuzumab, and the hazard for recurrence
went up at the end of the first year when trastuzumab was stopped. It may not
turn out to be anything, but I thought it was provocative. That was the most
interesting slide Ian Smith showed.
CD 2, Track 17
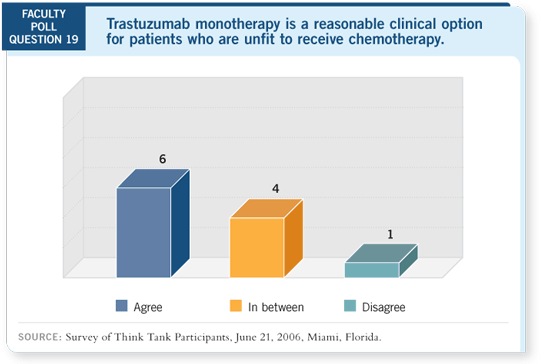
 DR LOVE: Craig, do you think trastuzumab without chemotherapy has a
role for the older, frail patient?
DR LOVE: Craig, do you think trastuzumab without chemotherapy has a
role for the older, frail patient?
 DR HENDERSON: I would be willing to consider it, but I certainly wouldn’t
give it much credence. I believe the data on the synergy of trastuzumab and
chemotherapy, both preclinical and clinical, are too compelling to spend much
time on that for the vast majority of patients.
DR HENDERSON: I would be willing to consider it, but I certainly wouldn’t
give it much credence. I believe the data on the synergy of trastuzumab and
chemotherapy, both preclinical and clinical, are too compelling to spend much
time on that for the vast majority of patients.
 DR LOVE: Chuck, for an 82-year-old patient with an ER-negative, PR-negative,
HER2-positive tumor, would you present the option of trastuzumab
monotherapy?
DR LOVE: Chuck, for an 82-year-old patient with an ER-negative, PR-negative,
HER2-positive tumor, would you present the option of trastuzumab
monotherapy?
 DR GEYER: I don’t believe that’s unreasonable. Again, I would have to be
convinced that chemotherapy was not indicated for that patient.
DR GEYER: I don’t believe that’s unreasonable. Again, I would have to be
convinced that chemotherapy was not indicated for that patient.
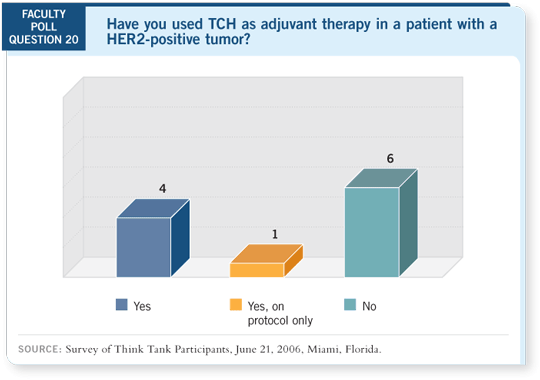
 DR WOLFF: One way to avoid this problem for an elderly person, for whom
you’re concerned about the potential for cardiac toxicity, might be to use
a nonanthracycline regimen. You could use TCH, for which we have data
(Slamon 2005).
DR WOLFF: One way to avoid this problem for an elderly person, for whom
you’re concerned about the potential for cardiac toxicity, might be to use
a nonanthracycline regimen. You could use TCH, for which we have data
(Slamon 2005).
Or, if you’re concerned about the toxicity associated with the higher doses of
docetaxel, you may create a regimen. One I can think of is weekly paclitaxel for 12 weeks with trastuzumab, which is highly tolerable in most patients.
You may be obtaining synergism from the chemotherapy/trastuzumab combination
and avoiding the anthracycline cardiotoxicity, although we have no
data for that.
 DR CHLEBOWSKI: We tried to use TCH for an 83-year-old woman who had a
7-cm fungating lesion with palpable lymphadenopathy. She didn’t tolerate the
chemotherapy, and we stopped it. Her tumor was shrinking, so we continued
with three more cycles of trastuzumab.
DR CHLEBOWSKI: We tried to use TCH for an 83-year-old woman who had a
7-cm fungating lesion with palpable lymphadenopathy. She didn’t tolerate the
chemotherapy, and we stopped it. Her tumor was shrinking, so we continued
with three more cycles of trastuzumab.
At surgery, she showed a pathological complete response in the breast and
lymph nodes. That’s the first time I’ve ever seen a patient with a fungating
lesion have a pathological complete response.
 DR LOVE: Joyce, have you used adjuvant trastuzumab as monotherapy?
DR LOVE: Joyce, have you used adjuvant trastuzumab as monotherapy?
 DR O’SHAUGHNESSY: I usually try to sneak in a little low-dose weekly
paclitaxel for patients with comorbidities. I saw a patient recently who was
extremely elderly, and I was considering an aromatase inhibitor and trastuzumab
alone for her.
DR O’SHAUGHNESSY: I usually try to sneak in a little low-dose weekly
paclitaxel for patients with comorbidities. I saw a patient recently who was
extremely elderly, and I was considering an aromatase inhibitor and trastuzumab
alone for her.
CD 2, Track 18
 DR GEYER: I must bring up Soon Paik’s data with cMYC, because the data
are remarkable. The patients with coamplified tumors who receive chemotherapy alone do very poorly, whereas we’re still waiting for a recurrence after
two years with the addition of trastuzumab. The separation of those curves
and their plateaus suggest that something important is going on. Among the
patients without cMYC amplification, we’re seeing the curves separate, but
they look like typical chemotherapy curves in that we’re continuing to see
recurrences even with trastuzumab (Kim 2005).
DR GEYER: I must bring up Soon Paik’s data with cMYC, because the data
are remarkable. The patients with coamplified tumors who receive chemotherapy alone do very poorly, whereas we’re still waiting for a recurrence after
two years with the addition of trastuzumab. The separation of those curves
and their plateaus suggest that something important is going on. Among the
patients without cMYC amplification, we’re seeing the curves separate, but
they look like typical chemotherapy curves in that we’re continuing to see
recurrences even with trastuzumab (Kim 2005).
CD 2, Track 19
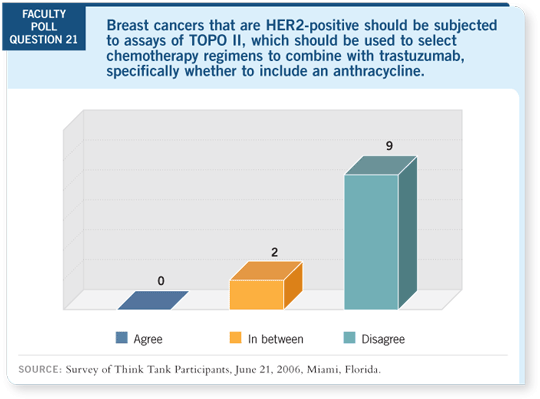
 DR LOVE: Debu, is the TOPO II assay something that could be considered
in clinical decision-making at this point?
DR LOVE: Debu, is the TOPO II assay something that could be considered
in clinical decision-making at this point?
 DR TRIPATHY: We’ve known for years that TOPO II overexpression confers
sensitivity to anthracyclines. This has been shown in cell-line models dating
back 10 to 15 years. Now we see that TOPO II amplification seems to predict
a better response to anthracyclines, and the lack of TOPO II amplification
might mean that anthracyclines are not as important (Slamon 2005; Press
2005).
DR TRIPATHY: We’ve known for years that TOPO II overexpression confers
sensitivity to anthracyclines. This has been shown in cell-line models dating
back 10 to 15 years. Now we see that TOPO II amplification seems to predict
a better response to anthracyclines, and the lack of TOPO II amplification
might mean that anthracyclines are not as important (Slamon 2005; Press
2005).
TOPO II is carrying more weight now. I would like to see the results from
BCIRG 006 replicated in data sets from HERA and the NSABP and Intergroup
studies. That should be imminently doable, and from what I understand,
plans are underway.
My guess is that TOPO II amplification will end up being significant. I don’t
believe it’s the only factor, but I do believe that it will enter into clinical
utility.
I expect cases will arise in which we want to withhold an anthracycline and
maybe use a taxane and trastuzumab. It’s not ready for prime time use, but it
should be soon.
Select publications

