
Select Excerpts from the Discussion
CD 1, Track 2
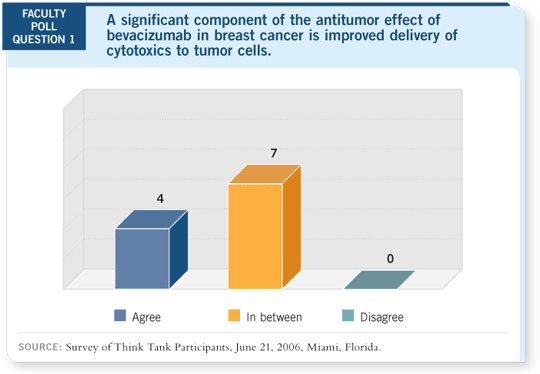
 DR TRIPATHY: Angiogenesis is an important target in cancer. It’s driven by
interactions between several receptors — including the VEGF receptor, the
one we are most familiar with, as well as an elaboration of proteases. So interrupting
this pathway is quite a task.
DR TRIPATHY: Angiogenesis is an important target in cancer. It’s driven by
interactions between several receptors — including the VEGF receptor, the
one we are most familiar with, as well as an elaboration of proteases. So interrupting
this pathway is quite a task.
An interesting correlative science study published recently by Sandy Swain’s
group at the NCI examined a variety of imaging and tissue indices for patients
with locally advanced and, primarily, inf lammatory breast cancer (Wedam
2006).
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was
performed at multiple time points — after the first cycle with bevacizumab
alone and following chemotherapy with bevacizumab. The imaging indices
demonstrated a drop in numerous DCE-MRI-based analyses, such as the
efflux of contrast, but indicated no difference between the patients who were
responders and those who were nonresponders (Wedam 2006).
What was interesting was that VEGFR2 (vascular endothelial growth factor
receptor 2) levels decreased, especially the phosphorylated or activated version,
and the localization of the receptors moved from the membrane to the
cytoplasm (Wedam 2006).
CD 1, Tracks 3, 5
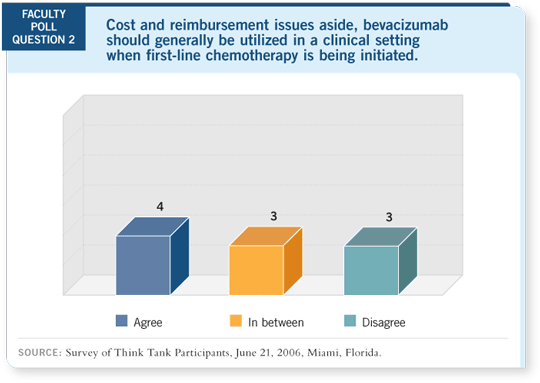
 DR LOVE: Putting cost and reimbursement issues aside, do we have
the data right now to justify using bevacizumab as first-line therapy
for metastatic disease, and how much of an advance was ECOG-E2100
(Miller 2005a, 2005b)?
DR LOVE: Putting cost and reimbursement issues aside, do we have
the data right now to justify using bevacizumab as first-line therapy
for metastatic disease, and how much of an advance was ECOG-E2100
(Miller 2005a, 2005b)?
 DR RUGO: It’s a significant advance. Having participated in the initial
capecitabine/bevacizumab trial (Miller 2005c) and also having used bevacizumab
in a variety of clinical research settings, we’ve been convinced for a
long time that it has clinical benefit.
DR RUGO: It’s a significant advance. Having participated in the initial
capecitabine/bevacizumab trial (Miller 2005c) and also having used bevacizumab
in a variety of clinical research settings, we’ve been convinced for a
long time that it has clinical benefit.
ECOG-E2100 produced two important implications (Miller 2005a, 2005b).
One is that we can, potentially, help patients in the metastatic setting with
first-line therapy in combination with a taxane.
The second is that it allowed us to move bevacizumab into trials in the early
adjuvant setting, as well as into the neoadjuvant setting, which potentially allows us to identify the patient population most likely to benefit from bevacizumab.
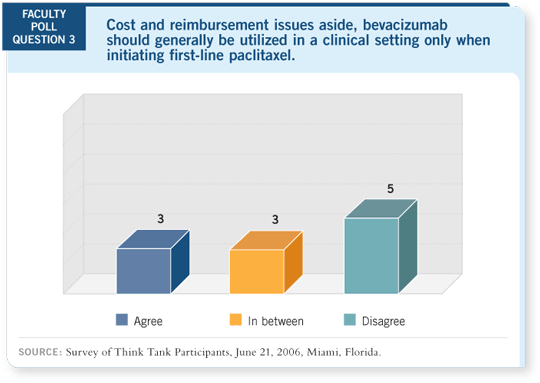
 DR LOVE: Bill, what are your thoughts about using bevacizumab in a clinical
setting?
DR LOVE: Bill, what are your thoughts about using bevacizumab in a clinical
setting?
 DR GRADISHAR: I believe clinicians will ultimately base their decisions on
what the data show. Right now, we have positive data from ECOG-E2100
(Miller 2005a, 2005b). By that, I’m emphasizing the fact that it’s used in the
first-line setting.
DR GRADISHAR: I believe clinicians will ultimately base their decisions on
what the data show. Right now, we have positive data from ECOG-E2100
(Miller 2005a, 2005b). By that, I’m emphasizing the fact that it’s used in the
first-line setting.
I have no reason to believe bevacizumab in conjunction with other agents, as
first-line therapy, wouldn’t have a similar benefit. I don’t believe we will see
people restricting themselves to the use of bevacizumab with paclitaxel alone,
but we don’t have a lot of Phase II data for combining bevacizumab with a
variety of different agents.
That said, the experience with trastuzumab is similar — we had preclinical
models that guided us and then the Phase II trials followed. They all were
consistent in that they demonstrated an incremental improvement when you
combined the given agent with trastuzumab. I believe that when bevacizumab
is combined with other chemotherapy agents, we will see the same improvement
in outcome that we’ve seen in ECOG-E2100.
CD 1, Track 7
 DR LOVE: Cliff, are you using capecitabine/bevacizumab off protocol?
DR LOVE: Cliff, are you using capecitabine/bevacizumab off protocol?
 DR HUDIS: I believe any patient with Stage IV breast cancer who is healthy
enough to receive bevacizumab deserves a shot at the drug. I don’t buy the
argument that it only works in the first-line setting and that it only works
with paclitaxel.
DR HUDIS: I believe any patient with Stage IV breast cancer who is healthy
enough to receive bevacizumab deserves a shot at the drug. I don’t buy the
argument that it only works in the first-line setting and that it only works
with paclitaxel.
The reasons I say that are, first, the drug has been extensively used with a
variety of other chemotherapy agents. I don’t have to see safety data for a drug
specifically in patients with breast cancer to call it safe. We have a lot of safety
data for the 5-FU/bevacizumab combination.
Second, I thought the capecitabine/bevacizumab trial by Dr Miller was a
positive signal. It showed a doubling of the response rate, but it did not achieve
its primary endpoint of progression-free survival (Miller 2005c).
The third reason is that you can see two patients in a clinic who are both
ready to receive first-line therapy but have extraordinarily different prior
chemotherapy experiences and exposure. For all these reasons, I offer bevacizumab,
essentially, to all eligible patients with a line of therapy at some point
in time.
 DR RUGO: We need the data from the ongoing RIBBON-1 and RIBBON-
2 trials. The trials randomly assign patients either in the first- or second-line
setting to receive chemotherapy with placebo or bevacizumab, and then they
allow a crossover. The potential exists to obtain a lot of information. We have
a menu of chemotherapy agents to choose from in those settings.
DR RUGO: We need the data from the ongoing RIBBON-1 and RIBBON-
2 trials. The trials randomly assign patients either in the first- or second-line
setting to receive chemotherapy with placebo or bevacizumab, and then they
allow a crossover. The potential exists to obtain a lot of information. We have
a menu of chemotherapy agents to choose from in those settings.
Select publications

