
Select Excerpts from the Discussion
CD 1, Track 21
 DR HUDIS: Everybody wants to enter into a debate about TAC and dose-dense
therapy. The first issue is that both of the regimens are clearly more
active than what they were compared against (Hudis 2005; Martin 2005).
DR HUDIS: Everybody wants to enter into a debate about TAC and dose-dense
therapy. The first issue is that both of the regimens are clearly more
active than what they were compared against (Hudis 2005; Martin 2005).
A practical study evaluating this issue is NSABP-B-38, which Sandy Swain
is chairing. Patients are randomly assigned to TAC, dose-dense AC  paclitaxel
or a third arm incorporating gemcitabine with dose-dense therapy. It’s
comparing six versus four cycles, every two-week versus every three-week
schedules and different taxanes and different anthracycline doses.
paclitaxel
or a third arm incorporating gemcitabine with dose-dense therapy. It’s
comparing six versus four cycles, every two-week versus every three-week
schedules and different taxanes and different anthracycline doses.
CD 1, Track 25
 DR LOVE: Chuck, in NSABP-B-38, you’re seeing patients who receive
TAC, dose-dense AC
DR LOVE: Chuck, in NSABP-B-38, you’re seeing patients who receive
TAC, dose-dense AC paclitaxel and the experimental regimen. What are
your observations about quality of life, fatigue, et cetera in different arms?
paclitaxel and the experimental regimen. What are
your observations about quality of life, fatigue, et cetera in different arms?
 DR GEYER: In terms of toxicity, the regimens aren’t segregating to the degree
that we thought we might see. We’re not going to pick up differences in
Grade I/II fatigue with what we’re doing. Those things, certainly, you can see
in the clinic. However, with the major toxicities that are monitored on the
trial, we’re not seeing any apparent differences with the cytokine support that
everybody receives.
DR GEYER: In terms of toxicity, the regimens aren’t segregating to the degree
that we thought we might see. We’re not going to pick up differences in
Grade I/II fatigue with what we’re doing. Those things, certainly, you can see
in the clinic. However, with the major toxicities that are monitored on the
trial, we’re not seeing any apparent differences with the cytokine support that
everybody receives.
CD 1, Tracks 22-23
 DR LOVE: Cliff, can you review the article about the benefits of adjuvant
chemotherapy based on ER status that was published by Berry in JAMA?
DR LOVE: Cliff, can you review the article about the benefits of adjuvant
chemotherapy based on ER status that was published by Berry in JAMA?
 DR HUDIS: Don Berry retrospectively went through three trials (CALGB-
8541, CALGB-9344 and CALGB-9741). Then across those studies, he generated
hypotheses about the value of treatment (Berry 2006).
DR HUDIS: Don Berry retrospectively went through three trials (CALGB-
8541, CALGB-9344 and CALGB-9741). Then across those studies, he generated
hypotheses about the value of treatment (Berry 2006).
I differ with the coauthors on the issue of ER testing. I’m not confident that
our testing is of high enough quality to draw conclusions. Also, we did not
apply standard and consistent hormone therapy for these patients. You must
recognize that when trying to interpret and put this into practice.
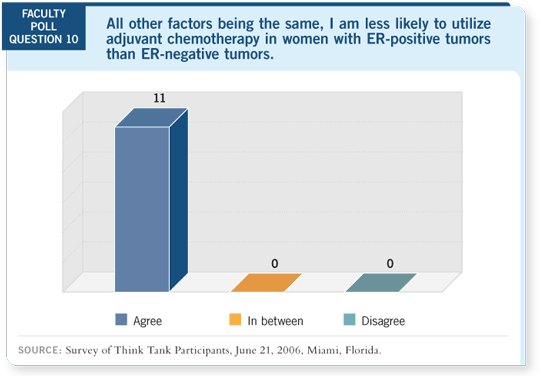
Among the patients with ER-negative disease, a significant benefit was
observed for high-dose versus low-dose CAF (CALGB-8541), paclitaxel versus
no paclitaxel (CALGB-9344) and every two-week versus every three-week
therapy (CALGB-9741). For the patients with ER-positive disease, the results
were quite different. No discernible advantage was observed for high-dose
CAF, although the low-dose CAF still appears to be inferior (Berry 2006).
Two take-home points have emerged. The first is that adjuvant chemotherapy
for patients with ER-negative breast cancer, both in terms of recurrence
rate and death, is a profoundly effective treatment that shouldn’t be scoffed
at. In terms of an indirect comparison, it is probably in the same ballpark as hormone therapy for patients with ER-positive disease and trastuzumab for
patients with HER2-positive disease.
For patients with ER-positive disease, the estimate of benefit at five years remains
favorable and above the threshold most patients would consider worthwhile. An
important caveat is that these data do not address whether chemotherapy works
in patients with ER-positive disease. It addresses the differences between chemotherapy
regimens in patients with ER-positive disease. You would need a chemotherapy-untreated control arm for the basis of comparison.
 DR LOVE: Debu, how do you factor in ER status when selecting adjuvant
chemotherapy for patients with node-positive disease?
DR LOVE: Debu, how do you factor in ER status when selecting adjuvant
chemotherapy for patients with node-positive disease?
 DR TRIPATHY: It’s clear that ER status is important. We’ve known this for
a long time, initially in the metastatic setting and then with the Oxford
overview (EBCTCG 2005), although it was never definitive because only a
small percentage of those patients had ER and PR testing. It’s not surprising
that we’re now finding that these patients derive a smaller benefit. They still
benefit, however, so that has to be factored in.
DR TRIPATHY: It’s clear that ER status is important. We’ve known this for
a long time, initially in the metastatic setting and then with the Oxford
overview (EBCTCG 2005), although it was never definitive because only a
small percentage of those patients had ER and PR testing. It’s not surprising
that we’re now finding that these patients derive a smaller benefit. They still
benefit, however, so that has to be factored in.
Some will argue that the low-dose version of CAF in 8541 was “no-dose”
chemotherapy, but that’s not technically true, and that’s one of the places
where, again, the data let us down. They don’t address that basic question:
Does chemotherapy work in this subset?
The explanation that Don put forth is that it’s about the hazard. This is more
important than just a little parlor game, because this speaks to the biology of
breast cancer. ER-negative disease has a more rapid proliferation early on,
and you see a high spike in recurrence risk, which is driven down by chemotherapy.
You don’t see that in patients with ER-positive disease because they don’t have
the early high risk. In fact, over 20 years, their risk is fairly consistent. This is
the sobering news about ER-positive disease. Even though you get through
the first five years doesn’t mean that you’re really “out of the woods.” It is
quite the opposite.
Should we change the numbers we currently use for the risk reduction associated
with chemotherapy and assign a different number to those patients with
ER-positive versus ER-negative disease? I believe we should, and incorporate
them into the model.
With regard to dose-dense therapy in combination with trastuzumab, I’m
still using the every three-week regimen used in the Intergroup and NSABP
studies (Romond 2005). Certainly, as more safety data come in and we have
more follow-up from the Memorial Sloan-Kettering study, I’m going to incorporate
that — just like many other changes in my practice — slowly.
 It’s interesting that the cardiotoxicity may be a little bit lower, although statistically
we can’t confirm that. I always projected that we might see slightly more cardiotoxicity when we
used a dose-dense anthracycline,
but that simply has not
been seen. The trend is in the
other direction (Hudis 2005).
It’s interesting that the cardiotoxicity may be a little bit lower, although statistically
we can’t confirm that. I always projected that we might see slightly more cardiotoxicity when we
used a dose-dense anthracycline,
but that simply has not
been seen. The trend is in the
other direction (Hudis 2005).
CD 1, Track 26
 DR HENDERSON: CALGB-9344 was designed initially to
evaluate three different doses
of doxorubicin, and paclitaxel
was an add-on. This created
a confounded study in many
ways. The paclitaxel issue,
however, became the positive
and the more provocative one
(Henderson 2003).
DR HENDERSON: CALGB-9344 was designed initially to
evaluate three different doses
of doxorubicin, and paclitaxel
was an add-on. This created
a confounded study in many
ways. The paclitaxel issue,
however, became the positive
and the more provocative one
(Henderson 2003).
We did an unplanned
subset analysis evaluating
the effects of adding paclitaxel for all patients. A highly significant reduction
was observed in the hazard ratio in favor of the taxane group. In the subset
of patients who had ER-positive disease, it was not significant. Among the
patients with ER-negative disease, the hazard reduction was highly significant
and larger than in the overall group (Henderson 2003).
Among the patients with HER2-negative disease, we found little evidence of
benefit from paclitaxel compared to the patients with HER2-positive disease,
for whom the benefit was much more robust.
We defined four subsets by HER2 and ER status. In the group with ER-negative
and HER2-negative disease, paclitaxel was advantageous. The
patients with ER-negative and HER2-positive disease showed a slightly
greater benefit (Hayes 2006).
For patients with ER-positive disease, as a whole, paclitaxel did not seem to confer
much of an advantage. In the small group of patients with ER-positive and HER2-
positive disease, a large advantage was associated with the addition of paclitaxel. Finally,
in the patients with ER-positive and HER2-negative disease, no advantage appeared
to be associated with the addition of paclitaxel (Hayes 2006).
Patients with ER-positive and HER2-negative tumors who were treated with
tamoxifen derived little or no benefit from the addition of paclitaxel. Is HER2
only predictive for paclitaxel or is this applicable to all cytotoxic therapies? My
bias — but, of course, we don’t have such data — is that this is not a paclitaxel
phenomenon but rather a chemotherapy phenomenon.
CD 2, Track 1
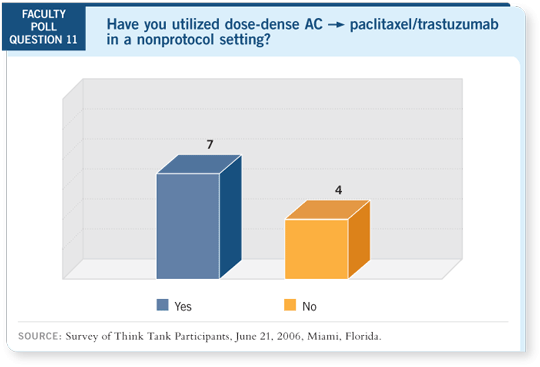
 DR LOVE: Hope, would you consider adjuvant chemotherapy for an
otherwise healthy woman in her eighties with triple-negative disease?
DR LOVE: Hope, would you consider adjuvant chemotherapy for an
otherwise healthy woman in her eighties with triple-negative disease?
 DR RUGO: Absolutely, but even more so for the patient with an ER-negative,
PR-negative and HER2-positive tumor, for whom we know that recurrence
is heavily weighted in the first two or three years. As the survival of our
population increases, these 81- and 82-year-old women who don’t have major
medical problems are reasonable candidates for limited approaches to chemotherapy.
DR RUGO: Absolutely, but even more so for the patient with an ER-negative,
PR-negative and HER2-positive tumor, for whom we know that recurrence
is heavily weighted in the first two or three years. As the survival of our
population increases, these 81- and 82-year-old women who don’t have major
medical problems are reasonable candidates for limited approaches to chemotherapy.
This must be within the limits that we all know to be important, such as
understanding morbidities. That’s one of the reasons Adjuvant! Online can be
very useful in directing physicians who are treating older patients. First, in this
older population, the patients with hormone receptor-negative disease are the
ones for whom we are going to be thinking about chemotherapy.
Then, in regard to morbidity, if a patient has a major morbidity, such as heart
failure, and is not going to be alive in three years, that is not the patient we
should be treating with chemotherapy.
 DR HENDERSON: We often make the assumption that dose-dense therapy is more toxic. If anything, it’s less toxic. Also, you shorten the duration of
chemotherapy, and for patients, this is not a minor issue. The older the patient,
the bigger the issue.
DR HENDERSON: We often make the assumption that dose-dense therapy is more toxic. If anything, it’s less toxic. Also, you shorten the duration of
chemotherapy, and for patients, this is not a minor issue. The older the patient,
the bigger the issue.
They don’t have as long a life expectancy, and they’re making a mental tradeoff:
“How much time am I going to have with a poor quality of life for a
benefit?” That’s the way they should be looking at it. So duration becomes
important. Every time we use a more intensive regimen, we assume it’s going
to be more toxic. That’s one of the surprising results of CALGB-9741. No
matter what we compare, it’s not necessarily more toxic and may be less so
(Citron 2003; Hudis 2005).
 DR HUDIS: In the quantifiable, objective ways in which we assess toxicity,
you cannot support the argument that dose-dense therapy is more toxic. In
CALGB-9741, it appears to be equivalent or perhaps less toxic in many ways.
The one toxicity that stood out in the original Citron paper was the high
rate of packed red blood cell transfusions (Citron 2003), which appears to be
abrogated with the use of erythropoietin or darbepoetin as prophylaxis.
DR HUDIS: In the quantifiable, objective ways in which we assess toxicity,
you cannot support the argument that dose-dense therapy is more toxic. In
CALGB-9741, it appears to be equivalent or perhaps less toxic in many ways.
The one toxicity that stood out in the original Citron paper was the high
rate of packed red blood cell transfusions (Citron 2003), which appears to be
abrogated with the use of erythropoietin or darbepoetin as prophylaxis.
It’s my subjective opinion that patients stay on schedule more easily when they
receive every two-week therapy with growth factor support than when they
are treated with an every three-week schedule. When patients can’t plan their
therapy, it is an annoyance, and it can reduce quality of life.
Also, completing therapy faster is always worthwhile. We’ve taken the position
that unless we have a compelling reason not to administer a growth factor, we
use every two-week therapy for everybody who receives AC and a taxane.
CD 2, Tracks 4-6
 DR LOVE: Antonio, can you discuss the study by Chuck Vogel evaluating
the use of pegfilgrastim for women with breast cancer?
DR LOVE: Antonio, can you discuss the study by Chuck Vogel evaluating
the use of pegfilgrastim for women with breast cancer?
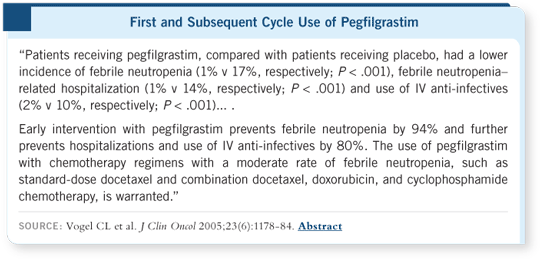
 DR WOLFF: The study results were published last year in the JCO. Patients
received docetaxel at 100 mg/m2 every 21 days with pegfilgrastim or placebo.
The data demonstrated that pegfilgrastim, compared to placebo, resulted in a
significant reduction in the risk of developing febrile neutropenia (17 versus
one percent), hospitalization (14 versus one percent) and intravenous antiinfective
use (10 versus two percent; [Vogel 2005]).
DR WOLFF: The study results were published last year in the JCO. Patients
received docetaxel at 100 mg/m2 every 21 days with pegfilgrastim or placebo.
The data demonstrated that pegfilgrastim, compared to placebo, resulted in a
significant reduction in the risk of developing febrile neutropenia (17 versus
one percent), hospitalization (14 versus one percent) and intravenous antiinfective
use (10 versus two percent; [Vogel 2005]).
 DR LOVE: Can you review the current guidelines for the use of growth factor
support?
DR LOVE: Can you review the current guidelines for the use of growth factor
support?
 DR WOLFF: Among the commonly used regimens in North America, those
with less than a 10 percent risk of febrile neutropenia include AC, CMF and
AC followed by paclitaxel every 21 days. The regimens with an intermediate
risk (10 to 20 percent) of febrile neutropenia include CEF and the FEC 120. Those with a greater than 20 percent risk of febrile neutropenia include
doxorubicin/docetaxel (AT) and TAC (Aapro 2006).
DR WOLFF: Among the commonly used regimens in North America, those
with less than a 10 percent risk of febrile neutropenia include AC, CMF and
AC followed by paclitaxel every 21 days. The regimens with an intermediate
risk (10 to 20 percent) of febrile neutropenia include CEF and the FEC 120. Those with a greater than 20 percent risk of febrile neutropenia include
doxorubicin/docetaxel (AT) and TAC (Aapro 2006).
Three clinical practice guidelines have been released in the last year: NCCN
(NCCN 2006), ASCO (Smith 2006) and EORTC (Aapro 2006). None
of these included a cost analysis because these are very difficult to do. The
recommendations are pretty uniform across the three practice guidelines when
it comes to the high-risk group — recommending the use of primary prophylaxis
with CSFs — and for the low-risk group (less than 10 percent risk of
febrile neutropenia) — not recommending the use of primary prophylaxis
with CSFs.
For the intermediate group, the recommendations are nuanced. The NCCN
would consider the use of prophylactic CSFs, and the ASCO guidelines would
not recommend them but would take into account special circumstances. The
EORTC would use individual risk factors.
 DR LOVE: Where did the threshold of 20 percent come from?
DR LOVE: Where did the threshold of 20 percent come from?
 DR WOLFF: For the ASCO committee, we had clear evidence from the Vogel
study that you were able to reduce the risk from approximately 20 percent
(Vogel 2005). I can quote what we wrote in the JCO article: “The 2005
Update Committee agreed unanimously that reduction in febrile neutropenia
was an important clinical outcome that justified the use of CSFs, regardless of impact on other factors — even economic factors — when the risk of
febrile neutropenia is approximately 20 percent and no other equally effective
regimen that does not require CSFs is available” (Smith 2006).
DR WOLFF: For the ASCO committee, we had clear evidence from the Vogel
study that you were able to reduce the risk from approximately 20 percent
(Vogel 2005). I can quote what we wrote in the JCO article: “The 2005
Update Committee agreed unanimously that reduction in febrile neutropenia
was an important clinical outcome that justified the use of CSFs, regardless of impact on other factors — even economic factors — when the risk of
febrile neutropenia is approximately 20 percent and no other equally effective
regimen that does not require CSFs is available” (Smith 2006).
The Vogel study included patients with metastatic and early-stage disease.
About two thirds of the patients had metastatic disease, and they might have
been more sensitive to the risk of neutropenia because of prior exposure to
chemotherapy (Vogel 2005). If a study had shown a reduction in the incidence
of febrile neutropenia from 10 percent to one percent, it is possible that the
threshold might have been listed at 10 percent.
Select publications

