
Select Excerpts from the Discussion
CD 2, Tracks 8-10
 DR LOVE: Antonio, can you comment on the two presentations made at
ASCO 2006 evaluating the cardiac toxicity associated with anthracyclines?
DR LOVE: Antonio, can you comment on the two presentations made at
ASCO 2006 evaluating the cardiac toxicity associated with anthracyclines?
 DR WOLFF: Sharon Giordano and her colleagues at MD Anderson conducted
a population-based observation study using the SEER-Medicare database. The
study included women 66 to 90 years of age who were diagnosed with breast
cancer from 1992 to 1999 and who had no other tumors or history of congestive
heart failure (CHF; [Giordano 2006]).
DR WOLFF: Sharon Giordano and her colleagues at MD Anderson conducted
a population-based observation study using the SEER-Medicare database. The
study included women 66 to 90 years of age who were diagnosed with breast
cancer from 1992 to 1999 and who had no other tumors or history of congestive
heart failure (CHF; [Giordano 2006]).
They ultimately identified about 31,000 women and separated them into three
groups: about 27,000 who received no adjuvant chemotherapy, about 2,300
who received nonanthracycline adjuvant chemotherapy and about 1,600 who
received anthracycline-based adjuvant chemotherapy. The mean age of these
women was 75 years, and the mean follow-up was 68 months. The patients
who received an anthracycline had higher-risk tumors (Giordano 2006).
They separated the patients into two specific age groups — 66 to 70 years and
71 to 90 years — and evaluated the 10-year outcomes in terms of the diagnosis
of CHF using ICD9 codes.
They observed that women aged 66 to 70 years who did not receive adjuvant
chemotherapy had a 73 percent chance of being free of CHF, whereas those
who received chemotherapy without an anthracycline had a 74 percent chance
(Giordano 2006).
On the other hand, those who received an anthracycline had a 61 percent
chance of being free of CHF, which is a 13 percent absolute difference at
10 years (Giordano 2006). Factors such as hypertension, diabetes mellitus,
coronary artery disease and peripheral vascular disease were significant in
identifying patients at risk.
Among the women aged 71 to 90 years, no difference was seen in the
incidence of CHF. The question was whether there was a selection bias or
whether those patients receiving an anthracycline had lower doses of chemotherapy.
However, individual records for those patients were not available.
The Shepherd study was an analysis of the MA5 trial, which included 710 pre- or
perimenopausal women with node-positive breast cancer who received CEF
with a cumulative epirubicin dose of 720 mg/m2 or CMF.
These women received strict follow-up in terms of LVEF, which was measured
at baseline, six, 12, 36 and 60 months (Shepherd 2006).
At the end of 60 months, 25 percent of the women treated with CEF had
at least a 10 percent absolute decrease in ejection fraction, whereas approximately
nine percent of the women treated with CMF had at least a 10 percent
decrease.
When they assessed the women with a greater than 20 percent decrease in
LVEF, the incidence was five percent in the CEF group and less than one
percent in the CMF group (Shepherd 2006).
In terms of the number of women with an LVEF that went below 50 percent,
the decrease was 17 percent for the CEF group and two percent for the CMF
group.
Among the women who had a greater than 20 percent reduction in LVEF,
only four cases of Grade III/IV CHF in the CEF group and no cases in the
CMF group were observed (Shepherd 2006). Hence, what exactly is the longterm
significance of these reductions in ejection fraction if most of them are
asymptomatic?
 DR HENDERSON: We have to be very careful with these data. The presentation
from Shepherd is compelling and would be the basis of my recommendations.
Ideally, these numbers should be risk adjusted, but I don’t believe they
were. The Giordano study, however, is misleading.
DR HENDERSON: We have to be very careful with these data. The presentation
from Shepherd is compelling and would be the basis of my recommendations.
Ideally, these numbers should be risk adjusted, but I don’t believe they
were. The Giordano study, however, is misleading.
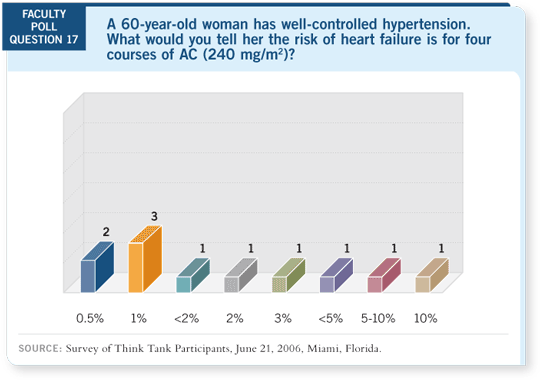
 DR LOVE: What would you estimate the risk of heart failure to be for a 60-year-old woman with well-controlled hypertension who receives four courses
of AC?
DR LOVE: What would you estimate the risk of heart failure to be for a 60-year-old woman with well-controlled hypertension who receives four courses
of AC?
 DR HENDERSON: I would use about two percent as my estimate. I don’t
know what to say about the decrease in LVEF. You need to explain that to the
patient, and you need to discuss both of those numbers. Obviously, CHF is the
important one, but with the high frequency of LVEF decline, the story isn’t
over yet. You have to assume that if you follow these patients another 10 years,
the one percent is going to become two or three percent.
DR HENDERSON: I would use about two percent as my estimate. I don’t
know what to say about the decrease in LVEF. You need to explain that to the
patient, and you need to discuss both of those numbers. Obviously, CHF is the
important one, but with the high frequency of LVEF decline, the story isn’t
over yet. You have to assume that if you follow these patients another 10 years,
the one percent is going to become two or three percent.
 DR RUGO: Based on the epirubicin data (Shepherd 2006), I’m still unclear
about whether we would tell somebody who’s receiving four cycles of AC that
they have a greater than one percent risk of cardiac damage. I don’t believe we
know how to judge the cardiac-damage risk for patients with well-controlled,
mild hypertension.
DR RUGO: Based on the epirubicin data (Shepherd 2006), I’m still unclear
about whether we would tell somebody who’s receiving four cycles of AC that
they have a greater than one percent risk of cardiac damage. I don’t believe we
know how to judge the cardiac-damage risk for patients with well-controlled,
mild hypertension.
Again, we’re not sure that hypertension, as a preexisting condition, increases
the long-term risk of CHF from an anthracycline. We don’t know that from
the Medicare database yet. It seems to me that one percent is a reasonable
number.
 DR GEYER: I had answered 0.5 percent based on the NSABP toxicity tables
and assuming you meant Grade III/IV heart failure. That’s key to answering
that question. Grade III/IV toxicity is consistently around 0.5 percent for four
courses of AC. I have been telling patients, since watching the NSABP-B-31
cardiac data for AC
DR GEYER: I had answered 0.5 percent based on the NSABP toxicity tables
and assuming you meant Grade III/IV heart failure. That’s key to answering
that question. Grade III/IV toxicity is consistently around 0.5 percent for four
courses of AC. I have been telling patients, since watching the NSABP-B-31
cardiac data for AC paclitaxel, that their chance of having a decline in their
ejection fraction is around five or six percent.
paclitaxel, that their chance of having a decline in their
ejection fraction is around five or six percent.
This is why I like that we’re backing off using chemotherapy for our patients
with node-negative disease. I’ve been worrying about the use of anthracyclines
for patients with ER-positive disease. I was the last person to stop using CMF
in the United States because of my concern about anthracycline cardiotoxicity.
CD 2, Track 12
 DR LOVE: Joyce, in terms of nonanthracycline options, can you discuss
the US Oncology trial Steve Jones reported comparing docetaxel/cyclophosphamide
(TC) to AC in the adjuvant setting?
DR LOVE: Joyce, in terms of nonanthracycline options, can you discuss
the US Oncology trial Steve Jones reported comparing docetaxel/cyclophosphamide
(TC) to AC in the adjuvant setting?
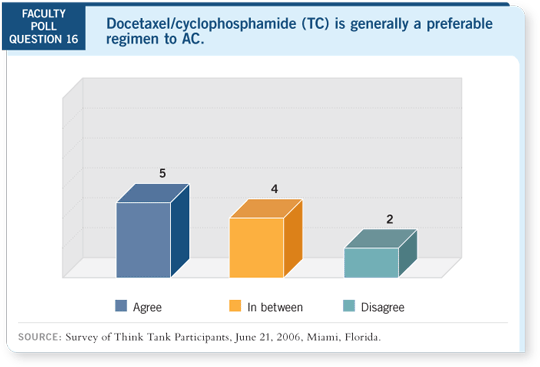
 DR O’SHAUGHNESSY: It was a randomized trial comparing four cycles of
either AC or TC with docetaxel at 75 mg/m2. The bottom line at five years
for disease-free survival, the primary endpoint, was 86 percent with TC and
80 percent with AC for a hazard ratio of 0.67 and a p-value of 0.015.
DR O’SHAUGHNESSY: It was a randomized trial comparing four cycles of
either AC or TC with docetaxel at 75 mg/m2. The bottom line at five years
for disease-free survival, the primary endpoint, was 86 percent with TC and
80 percent with AC for a hazard ratio of 0.67 and a p-value of 0.015.
For overall survival, a three percent absolute improvement at five years in
favor of the TC was observed. The hazard ratio was 0.76, which was trending
toward significance but not statistical significance ( Jones 2005).
Steve’s concluding slide stated that TC should now be considered a standard nonanthracycline adjuvant regimen for patients with operable breast cancer
( Jones 2005). I agree. In my practice, I don’t use AC anymore as a four-cycle
regimen. I’ve switched over to TC. When I’m going to use four cycles for
patients with lower-risk, ER-positive, node-negative disease, I use TC.
CD 2, Track 13
 DR LOVE: Debu, what are your thoughts about nonanthracycline
regimens?
DR LOVE: Debu, what are your thoughts about nonanthracycline
regimens?
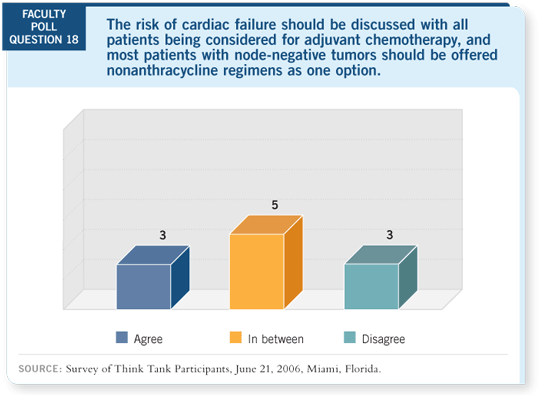
 DR TRIPATHY: Cardiac issues are clearly important, and we underestimate this
risk. After decades of attention to heart disease in this country, it’s no surprise
that patients are concerned. So we need regimens that are less cardiotoxic.
DR TRIPATHY: Cardiac issues are clearly important, and we underestimate this
risk. After decades of attention to heart disease in this country, it’s no surprise
that patients are concerned. So we need regimens that are less cardiotoxic.
We don’t know the long-term history for cardiac toxicity because we’ve never
measured it systematically and it’s a hard thing to measure. When you consider
population-based studies, you may overestimate it because a lot of people are
probably misclassified. When you consider clinical trials, you may underestimate
it because of selection bias of the patients enrolled.
The TC regimen is a reasonable nonanthracycline regimen. It was a smaller
study, so our level of confidence is lower, but at least everything pointed in the
right direction. Despite the limitations of being a small study, being spread out
over a large period of time and having broad eligibility criteria, it did show
that TC is as good or probably even better than AC ( Jones 2005). For patients
with cardiac risk factors, it’s a reasonable regimen to use.
 DR LOVE: For patients who don’t have cardiac risk factors, do you think TC
should be brought up as an option?
DR LOVE: For patients who don’t have cardiac risk factors, do you think TC
should be brought up as an option?
 DR TRIPATHY: I believe it can be. When I look at the overall toxicity profile,
it’s hard for me to figure out which was better tolerated because the spectrum
of toxicities was different. The TC regimen had more hematologic toxicities
and edema, whereas the AC regimen had more GI and cardiac toxicities.
DR TRIPATHY: I believe it can be. When I look at the overall toxicity profile,
it’s hard for me to figure out which was better tolerated because the spectrum
of toxicities was different. The TC regimen had more hematologic toxicities
and edema, whereas the AC regimen had more GI and cardiac toxicities.
 DR O’SHAUGHNESSY: We observed one case of CHF in the AC arm and zero
in the TC arm. We didn’t do a lot of monitoring.
DR O’SHAUGHNESSY: We observed one case of CHF in the AC arm and zero
in the TC arm. We didn’t do a lot of monitoring.
 DR LOVE: Peter, what are your thoughts about TC?
DR LOVE: Peter, what are your thoughts about TC?
 DR RAVDIN: If we had started with TC, we wouldn’t be going across to AC
based on the results of this trial. The US Oncology trial is of modest size, and
it shows a statistically significant disease-free survival and the same size overall
survival. It’s simply underpowered to make statistical significance ( Jones
2005).
DR RAVDIN: If we had started with TC, we wouldn’t be going across to AC
based on the results of this trial. The US Oncology trial is of modest size, and
it shows a statistically significant disease-free survival and the same size overall
survival. It’s simply underpowered to make statistical significance ( Jones
2005).
I would anticipate that, over the next couple of years, people who want to use a
short 12-week regimen will be using TC much more frequently than a year ago.
With regard to the cardiac toxicity, I agree that the SEER analysis with Medicare records wasn’t absolutely convincing. Among other things, the
numbers seemed high. I would like to know what those numbers are in a
general population, but we have a major problem with the ascertainment of
late toxicity in clinical trials.
Select publications

