
 |
||||||||||||||
| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1-2
![]() DR LOVE: Can you summarize what we currently know about neoadjuvant
endocrine therapy?
DR LOVE: Can you summarize what we currently know about neoadjuvant
endocrine therapy?
![]() PROF SMITH: Nearly all the work conducted with neoadjuvant endocrine
therapy has been with postmenopausal women. Three large trials have
compared the aromatase inhibitors to tamoxifen (Cataliotti 2006; Dowsett
2005; Ellis 2003; Smith 2005), all of which demonstrated that the aromatase
inhibitors are more effective in terms of tumor regression and reducing the
need for a mastectomy.
PROF SMITH: Nearly all the work conducted with neoadjuvant endocrine
therapy has been with postmenopausal women. Three large trials have
compared the aromatase inhibitors to tamoxifen (Cataliotti 2006; Dowsett
2005; Ellis 2003; Smith 2005), all of which demonstrated that the aromatase
inhibitors are more effective in terms of tumor regression and reducing the
need for a mastectomy.
![]() DR LOVE: How do you approach the choice between neoadjuvant chemotherapy
and endocrine therapy?
DR LOVE: How do you approach the choice between neoadjuvant chemotherapy
and endocrine therapy?
![]() PROF SMITH: That’s the real crunch. The more I administer neoadjuvant
endocrine therapy, the more I wonder why we don’t utilize it more frequently.
When it works, it’s extremely effective. Anecdotally, I recently treated a
woman in her sixties with a 5-cm, hormone receptor-positive tumor who did
not want to receive chemotherapy but wished to avoid mastectomy. I treated
her disease with an aromatase inhibitor, and she underwent breast-conserving
surgery with only a small, 1-cm residual tumor.
PROF SMITH: That’s the real crunch. The more I administer neoadjuvant
endocrine therapy, the more I wonder why we don’t utilize it more frequently.
When it works, it’s extremely effective. Anecdotally, I recently treated a
woman in her sixties with a 5-cm, hormone receptor-positive tumor who did
not want to receive chemotherapy but wished to avoid mastectomy. I treated
her disease with an aromatase inhibitor, and she underwent breast-conserving
surgery with only a small, 1-cm residual tumor.
The question was whether or not she needed chemotherapy. Until recently, no data existed to address this question, but we are beginning to evaluate our results from the IMPACT trial, which compared neoadjuvant anastrozole to tamoxifen. Matt Ellis is also evaluating the data from the P-024 trial of letrozole versus tamoxifen.
We are putting together an algorithm that suggests that patients with node-negative breast cancer, a good tumor response (smaller than one centimeter at surgery) and good suppression of Ki-67 while the tumor remains hormone receptor-positive have an excellent long-term outcome (Dowsett 2007). Patients with node-negative breast cancer at surgery with these parameters almost never experience relapse.
Track 3
![]() DR LOVE: Can you discuss the issue of extended adjuvant endocrine
therapy beyond five years?
DR LOVE: Can you discuss the issue of extended adjuvant endocrine
therapy beyond five years?
![]() PROF SMITH: The cleanest, most important data of the aromatase inhibitor
trials addressing this issue are from MA17, which demonstrated that patients
who had received tamoxifen for five years and were switched to letrozole fared
better than those who received placebo (Goss 2008; Ingle 2008). The evidence
suggests that the longer you treat beyond five years, the greater the benefit.
PROF SMITH: The cleanest, most important data of the aromatase inhibitor
trials addressing this issue are from MA17, which demonstrated that patients
who had received tamoxifen for five years and were switched to letrozole fared
better than those who received placebo (Goss 2008; Ingle 2008). The evidence
suggests that the longer you treat beyond five years, the greater the benefit.
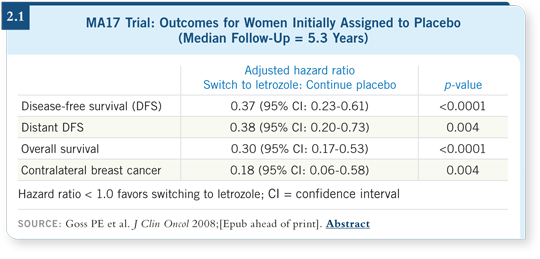
Another interesting aspect of MA17 is that when the results were first presented after two and a half years — because the benefit was more dramatic than imagined — patients on the placebo were offered the opportunity to switch. Some switched and some did not, but those who did had worse prognostic features in their original disease. Those patients are now faring better than the ones who didn’t switch, even though they had poorer prognoses (2.1). That’s a powerful message regarding the long-term use of aromatase inhibitors. Some women may need to receive these agents for an extended period of time.
Tracks 7-8
![]() DR LOVE: Can you provide an overview of neoadjuvant therapy for
patients with HER2-positive tumors?
DR LOVE: Can you provide an overview of neoadjuvant therapy for
patients with HER2-positive tumors?
![]() PROF SMITH: For surgeons dealing with large tumors, the most spectacular
data on trastuzumab are in the neoadjuvant setting. A small but influential MD Anderson study showed a pathologic complete response rate of approximately
60 percent with the use of trastuzumab in addition to neoadjuvant
chemotherapy (Buzdar 2007; [2.2]).
PROF SMITH: For surgeons dealing with large tumors, the most spectacular
data on trastuzumab are in the neoadjuvant setting. A small but influential MD Anderson study showed a pathologic complete response rate of approximately
60 percent with the use of trastuzumab in addition to neoadjuvant
chemotherapy (Buzdar 2007; [2.2]).
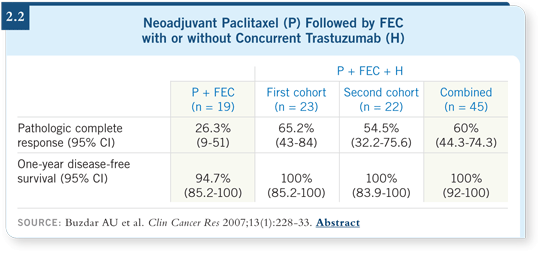
The results were almost too good to be true, but now a large European trial in inflammatory breast cancer (NOAH) has also demonstrated a high pathologic complete response rate with the addition of trastuzumab compared to neoadjuvant chemotherapy alone (Gianni 2007).
If a patient has a large, HER2-positive breast tumor and you are considering neoadjuvant treatment, then you must administer trastuzumab up front with the chemotherapy rather than waiting until after surgery.
Track 9
![]() DR LOVE: What is your approach for the patient with a node-negative,
HER2-positive tumor (Press 1997; [2.3])?
DR LOVE: What is your approach for the patient with a node-negative,
HER2-positive tumor (Press 1997; [2.3])?
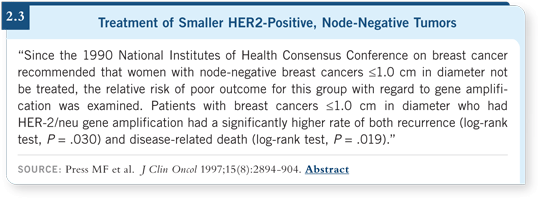
![]() PROF SMITH: The issue that’s beginning to emerge — and I’ve been
impressed because it’s changed my thinking — is that the prognosis with HER2-positive tumors of one centimeter or less is approximately a 15 to 20
percent risk of relapse within 10 years (Press 1997). So we probably need to be
more aggressive with these small, node-negative, HER2-positive tumors and
bias ourselves toward using chemotherapy and trastuzumab.
PROF SMITH: The issue that’s beginning to emerge — and I’ve been
impressed because it’s changed my thinking — is that the prognosis with HER2-positive tumors of one centimeter or less is approximately a 15 to 20
percent risk of relapse within 10 years (Press 1997). So we probably need to be
more aggressive with these small, node-negative, HER2-positive tumors and
bias ourselves toward using chemotherapy and trastuzumab.
| Table of Contents | Top of Page |
INTERVIEWS
Neil Love, MD
Editor
Monica Morrow, MD
- Select publications
Ian E Smith, MD
- Select publications
Robert W Carlson, MD
- Select publications
Soonmyung Paik, MD
- Select publications
Breast Cancer Update
for Surgeons:
A CME Audio Series and Activity