
 |
||||||||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 3-4
![]() DR LOVE: Where are we right now in terms of the risks and benefits of
aromatase inhibitors for postmenopausal women with breast cancer?
DR LOVE: Where are we right now in terms of the risks and benefits of
aromatase inhibitors for postmenopausal women with breast cancer?
![]() DR CARLSON: The 100-month follow-up of the ATAC trial was one of the
most important abstracts presented at San Antonio. The results were encouraging and reassuring. From the Early Breast Cancer Trialists’ Collaborative
Group (EBCTCG) analysis, we know that the benefits of tamoxifen, in terms
of risk reduction for recurrence and death, persist well beyond the period of
actual tamoxifen administration (EBCTCG 2005). Some were concerned that
this might not be the case with the aromatase inhibitors — that you might
win the short game but lose the long game.
DR CARLSON: The 100-month follow-up of the ATAC trial was one of the
most important abstracts presented at San Antonio. The results were encouraging and reassuring. From the Early Breast Cancer Trialists’ Collaborative
Group (EBCTCG) analysis, we know that the benefits of tamoxifen, in terms
of risk reduction for recurrence and death, persist well beyond the period of
actual tamoxifen administration (EBCTCG 2005). Some were concerned that
this might not be the case with the aromatase inhibitors — that you might
win the short game but lose the long game.
The efficacy data from the ATAC trial suggest substantial benefit from anastrozole beyond the five years of actual therapy (ATAC Trialists’ Group 2008; [3.1]). The long-term differences were larger in the ATAC trial than in the EBCTCG analysis of the tamoxifen carryover effect. It’s an indirect comparison, so we have to be cautious, but it is reassuring to observe sustained benefits from anastrozole after treatment is completed.
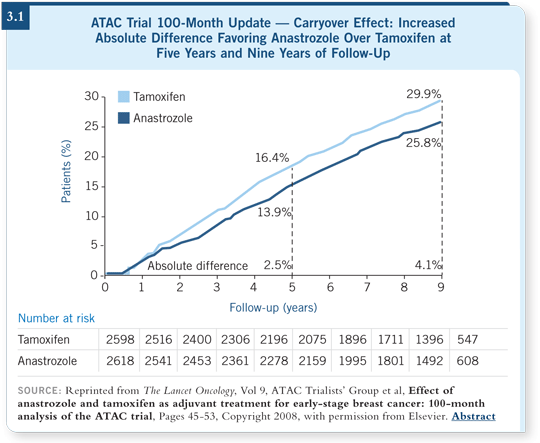
The toxicity data were also reassuring. No unexpected toxicities, especially bone events, were recorded on long-term follow-up (ATAC Trialists’ Group 2008; [3.1]).
Patients received the initial five years of anastrazole or tamoxifen, and in the subsequent five years, the fracture rates for the women treated with tamoxifen and those treated with anastrozole were superimposable.
![]() DR LOVE: We forget that these patients did not receive bone monitoring and
were not administered bisphosphonate therapy. Now that’s part of clinical
practice.
DR LOVE: We forget that these patients did not receive bone monitoring and
were not administered bisphosphonate therapy. Now that’s part of clinical
practice.
![]() DR CARLSON: One of the possible explanations for the fracture curves
coming together with the extended follow-up in the ATAC trial is that we
have learned that you need to evaluate bone health. Women in the aromatase
inhibitor arm may have had their bones assessed and may have received an off-protocol
intervention.
DR CARLSON: One of the possible explanations for the fracture curves
coming together with the extended follow-up in the ATAC trial is that we
have learned that you need to evaluate bone health. Women in the aromatase
inhibitor arm may have had their bones assessed and may have received an off-protocol
intervention.
![]() DR LOVE: That’s an interesting thought. Another important finding involved
the incidence of endometrial cancer during years five through nine: One case
versus 12 cases in the anastrozole and tamoxifen arms, respectively (ATAC
Trialists’ Group 2008). The presenters posed the question of whether the
absence of tamoxifen increases the risk or whether anastrozole has a preventive
effect on endometrial cancer, which doesn’t seem that far fetched. What are
your thoughts on this?
DR LOVE: That’s an interesting thought. Another important finding involved
the incidence of endometrial cancer during years five through nine: One case
versus 12 cases in the anastrozole and tamoxifen arms, respectively (ATAC
Trialists’ Group 2008). The presenters posed the question of whether the
absence of tamoxifen increases the risk or whether anastrozole has a preventive
effect on endometrial cancer, which doesn’t seem that far fetched. What are
your thoughts on this?
![]() DR CARLSON: It’s hard to sort out from the data we have, but one would
surmise from those numbers that it’s a little of both.
DR CARLSON: It’s hard to sort out from the data we have, but one would
surmise from those numbers that it’s a little of both.
Track 11
![]() DR LOVE: Can you describe how the Oncotype DX assay has influenced
your practice?
DR LOVE: Can you describe how the Oncotype DX assay has influenced
your practice?
![]() DR CARLSON: In my practice, I consider using it for women with ER-positive,
HER2-negative, lymph node-negative disease, especially in situations
in which the woman is reluctant to consider chemotherapy and when
the result of the assay would make a difference to her or to me in terms of the
confidence with which we approach the therapy.
DR CARLSON: In my practice, I consider using it for women with ER-positive,
HER2-negative, lymph node-negative disease, especially in situations
in which the woman is reluctant to consider chemotherapy and when
the result of the assay would make a difference to her or to me in terms of the
confidence with which we approach the therapy.
Women with T1A and probably T1B tumors fare well regardless of what the biomarkers show. It’s for the women who have the T1C, the 1-to 2-cm or even the 3-cm node-negative tumors, that we hope these newer biological systems will be helpful.
Track 14
![]() DR LOVE: Can you summarize what’s happened recently in terms of anti-HER2 therapy for patients with HER2-positive tumors?
DR LOVE: Can you summarize what’s happened recently in terms of anti-HER2 therapy for patients with HER2-positive tumors?
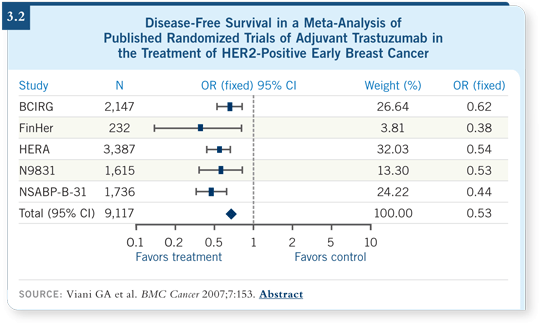
![]() DR CARLSON: We have seen a tremendous paradigm shift in how we
approach HER2-positive breast cancer, especially in the adjuvant setting. We
now have six or seven major randomized trials evaluating combination chemotherapy
with or without trastuzumab in the adjuvant setting. Those studies,
with the exception of one that was recently reported, are remarkably consistent
in the finding that the addition of trastuzumab decreases the risk of recurrence
by about 50 percent and decreases the risk of death from breast cancer by
about 35 percent (Smith 2007; Slamon 2006; Perez 2007; Viani 2007; [3.2]).
DR CARLSON: We have seen a tremendous paradigm shift in how we
approach HER2-positive breast cancer, especially in the adjuvant setting. We
now have six or seven major randomized trials evaluating combination chemotherapy
with or without trastuzumab in the adjuvant setting. Those studies,
with the exception of one that was recently reported, are remarkably consistent
in the finding that the addition of trastuzumab decreases the risk of recurrence
by about 50 percent and decreases the risk of death from breast cancer by
about 35 percent (Smith 2007; Slamon 2006; Perez 2007; Viani 2007; [3.2]).
Those are tremendous risk reductions, the types we see with endocrine therapy in hormone receptor-positive breast cancer. They have resulted in the rapid adoption of trastuzumab-containing adjuvant regimens in HER2-overexpressed breast cancer.
| Table of Contents | Top of Page |
INTERVIEWS
Neil Love, MD
Editor
Monica Morrow, MD
- Select publications
Ian E Smith, MD
- Select publications
Robert W Carlson, MD
- Select publications
Soonmyung Paik, MD
- Select publications
Breast Cancer Update
for Surgeons:
A CME Audio Series and Activity