
 |
||||||||||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3
![]() DR LOVE: What are your thoughts on chemoprevention and the STAR
trial (NSABP-P-2)?
DR LOVE: What are your thoughts on chemoprevention and the STAR
trial (NSABP-P-2)?
![]() DR MORROW: Chemoprevention is something that both funding agencies and
medical organizations have held as an important ideal, but in practice it hasn’t
come to pass.
DR MORROW: Chemoprevention is something that both funding agencies and
medical organizations have held as an important ideal, but in practice it hasn’t
come to pass.
We started with tamoxifen, a drug that produces a 50 percent risk reduction in the development of breast cancer in women who are at increased risk and approximately an 80 percent risk reduction in those who are at risk on the basis of atypical hyperplasia (Fisher 1998).
Because of tamoxifen’s side-effect profile, we never saw a wide uptake in its use by healthy women.
I found the results from the STAR trial — a direct comparison of tamoxifen and raloxifene in postmenopausal women at increased risk of developing breast cancer — to be exciting. Raloxifene was equivalent to tamoxifen as a chemoprevention agent, and it had a significantly improved side-effect profile (Vogel 2006; [1.1]).
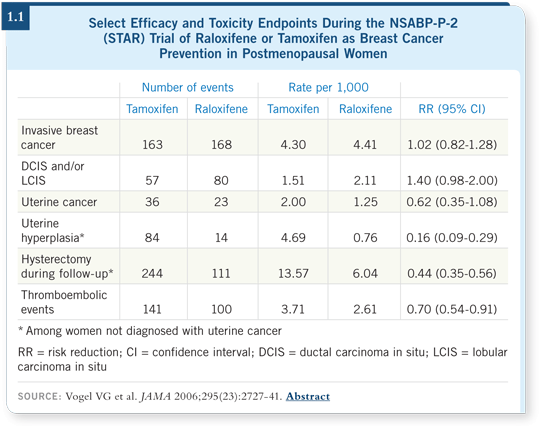
We saw no evidence of increased risk of endometrial cancer or deep vein thrombosis, but a beneficial antiosteoporosis effect was observed in patients treated with raloxifene.
I believe that any woman who has a biopsy that shows atypical hyperplasia or a patient who has more than one first-degree relative with breast cancer definitely needs to have a discussion about chemoprevention. For postmenopausal women who can receive the antiosteoporosis benefit, raloxifene is a win-win situation.
Track 4
![]() DR LOVE: What are your thoughts on the 100-month update of the
ATAC trial?
DR LOVE: What are your thoughts on the 100-month update of the
ATAC trial?
![]() DR MORROW: Clearly, the results are holding up long term. A question was
whether there would be a “carryover” effect with the aromatase inhibitors, as
we’ve seen with tamoxifen, in terms of the long-term reduction in contralateral
breast cancer and survival benefits.
DR MORROW: Clearly, the results are holding up long term. A question was
whether there would be a “carryover” effect with the aromatase inhibitors, as
we’ve seen with tamoxifen, in terms of the long-term reduction in contralateral
breast cancer and survival benefits.
The 100-month ATAC trial data suggest that the same carryover effect is present, and that’s reassuring (ATAC Trialists’ Group 2008; [3.1]).
The idea that the osteoporosis and fracture problems appear to stabilize over time is also reassuring (ATAC Trialists’ Group 2008), although it doesn’t obviate the increased risk of osteoporosis in the early treatment period. This needs to be monitored and is an issue in the chemoprevention setting.
Most of the side-effect profile of the aromatase inhibitors appears to be preferable to that of tamoxifen, with the exception of the bone and joint problems, which for some women can be significantly disabling.
Tracks 7-8
![]() DR LOVE: It is my understanding that the Oncotype DX assay is going
to start reporting quantitative ER. What are your thoughts on this
development?
DR LOVE: It is my understanding that the Oncotype DX assay is going
to start reporting quantitative ER. What are your thoughts on this
development?
![]() DR MORROW: Several studies suggest that when you use RT-PCR to
measure ER, you obtain a result that correlates better with response than if
you measure it by immunohistochemistry. When you have a single laboratory
engaged in quality control, you have a better chance of obtaining a valid
result.
DR MORROW: Several studies suggest that when you use RT-PCR to
measure ER, you obtain a result that correlates better with response than if
you measure it by immunohistochemistry. When you have a single laboratory
engaged in quality control, you have a better chance of obtaining a valid
result.
![]() DR LOVE: What is your opinion of the study evaluating the Oncotype DX
assay in patients with ER-positive, node-positive disease?
DR LOVE: What is your opinion of the study evaluating the Oncotype DX
assay in patients with ER-positive, node-positive disease?
![]() DR MORROW: I thought it was fascinating. For so long, node-positive disease
has been the hallmark of a bad outcome and more treatment. But in our
practices we have these patients, some of whom have had large numbers of
positive nodes, who are still alive 15 and 20 years later.
DR MORROW: I thought it was fascinating. For so long, node-positive disease
has been the hallmark of a bad outcome and more treatment. But in our
practices we have these patients, some of whom have had large numbers of
positive nodes, who are still alive 15 and 20 years later.
The Oncotype DX report indicates that the biology of the disease is equally diverse in patients with node-negative and node-positive disease and that a phenomenon of regional disease exists that is not necessarily systemic.
The level of the recurrence score may be a useful guide for the intensity of the chemotherapy needed, but it may not be the same for all patients with node-positive disease (Albain 2007; [4.2]).
Track 14
![]() DR LOVE: What are your thoughts on the role of sentinel lymph node
biopsy in the patient who has received neoadjuvant therapy?
DR LOVE: What are your thoughts on the role of sentinel lymph node
biopsy in the patient who has received neoadjuvant therapy?
![]() DR MORROW: That’s a controversial issue that we debated at a recent meeting
almost more than any other subject in local therapy. Neoadjuvant therapy will
reduce the incidence of positive nodes. So you’re saving women an axillary
dissection.
DR MORROW: That’s a controversial issue that we debated at a recent meeting
almost more than any other subject in local therapy. Neoadjuvant therapy will
reduce the incidence of positive nodes. So you’re saving women an axillary
dissection.
The data Terry Mamounas published from NSABP-B-27, which evaluated 428 patients who had a sentinel lymph node biopsy after neoadjuvant therapy, suggest that the accuracy rate is the same as it is for women who had a primary sentinel node biopsy (Mamounas 2005). Granted, we do not have long-term follow-up data on axillary failure rates in that population, but I am comfortable with that.
I am not comfortable with a sentinel lymph node biopsy for the patient who starts pretreatment with either a clinically positive axillary node or a node that is documented by needle biopsy to be positive but is downstaged to clinically node-negative after neoadjuvant therapy.
You have a higher false-negative rate in that population, and it may be as high as 20 or 30 percent. Most patients with one grossly positive node have other positive nodes, and the likelihood of an axillary pathologic complete response is only about 20 or 25 percent. Putting that together, I consider it an indication for axillary dissection.
| Table of Contents | Top of Page |
INTERVIEWS
Neil Love, MD
Editor
Monica Morrow, MD
- Select publications
Ian E Smith, MD
- Select publications
Robert W Carlson, MD
- Select publications
Soonmyung Paik, MD
- Select publications
Breast Cancer Update
for Surgeons:
A CME Audio Series and Activity