
 |
||||||||||||||

| Tracks 1-9 | ||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you comment on the current assays being used to evaluate
ER and HER2?
DR LOVE: Can you comment on the current assays being used to evaluate
ER and HER2?
![]() DR PAIK: The most important take-home lesson is that none of these tests is
perfect.
DR PAIK: The most important take-home lesson is that none of these tests is
perfect.
It is almost frightening to receive data from places where, depending on which day the tissue is processed or which operation was performed, the ER result changes. Tumors removed on Friday have a much lower rate of ER positivity compared to the rest because they were in formula longer — over the weekend — and were not immediately processed into the tissue block.
![]() DR LOVE: Is that relevant mainly to IHC testing?
DR LOVE: Is that relevant mainly to IHC testing?
![]() DR PAIK: Yes. Unfortunately the ER assessment is done by IHC. This
problem has fewer implications for FISH testing of HER2.
DR PAIK: Yes. Unfortunately the ER assessment is done by IHC. This
problem has fewer implications for FISH testing of HER2.
![]() DR LOVE: How does a surgeon in a community hospital ensure that a patient
will have appropriate ER and HER2 testing?
DR LOVE: How does a surgeon in a community hospital ensure that a patient
will have appropriate ER and HER2 testing?
![]() DR PAIK: Surgeons have a duty to communicate with the pathology department
to demand quality control and quality assurance data. They have to understand
which test is used at that particular lab. Is it reliable? It has to meet certain
standards. For example, for HER2, I believe testing must meet the ASCO/CAP
testing guideline (Wolff 2007). The quality control checks must be made.
DR PAIK: Surgeons have a duty to communicate with the pathology department
to demand quality control and quality assurance data. They have to understand
which test is used at that particular lab. Is it reliable? It has to meet certain
standards. For example, for HER2, I believe testing must meet the ASCO/CAP
testing guideline (Wolff 2007). The quality control checks must be made.
![]() DR LOVE: How do you check on certification?
DR LOVE: How do you check on certification?
![]() DR PAIK: For HER2 testing, CAP will enforce the quality control beginning
this year. If labs cannot meet the certification requirements, they are not
supposed to perform the HER2 test.
DR PAIK: For HER2 testing, CAP will enforce the quality control beginning
this year. If labs cannot meet the certification requirements, they are not
supposed to perform the HER2 test.
Track 7
![]() DR LOVE: Can you discuss your work with the Oncotype DX assay and
its role in clinical decision-making regarding the use of adjuvant chemotherapy?
DR LOVE: Can you discuss your work with the Oncotype DX assay and
its role in clinical decision-making regarding the use of adjuvant chemotherapy?
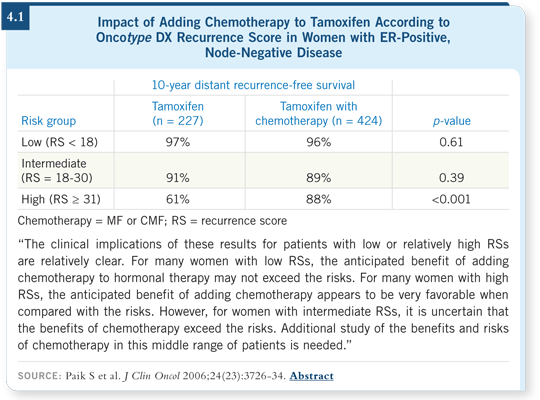
![]() DR PAIK: When we examined the gene list that the Oncotype DX assay
comprised, we realized that it was heavily populated by ER-related and proliferation-related genes. We hypothesized that this test might be predictive of
chemotherapy response (Paik 2006; [4.1]).
DR PAIK: When we examined the gene list that the Oncotype DX assay
comprised, we realized that it was heavily populated by ER-related and proliferation-related genes. We hypothesized that this test might be predictive of
chemotherapy response (Paik 2006; [4.1]).
![]() DR LOVE: The Oncotype DX assay has been integrated into the clinical
management of the node-negative tumor. But in the last few months, we’ve
begun to see data emerge from patients with node-positive tumors. Can you
talk about what’s been observed?
DR LOVE: The Oncotype DX assay has been integrated into the clinical
management of the node-negative tumor. But in the last few months, we’ve
begun to see data emerge from patients with node-positive tumors. Can you
talk about what’s been observed?
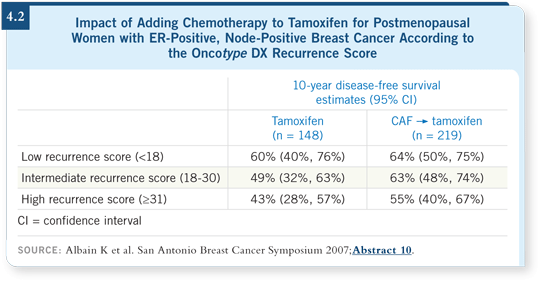
![]() DR PAIK: The SWOG study Dr Kathy Albain presented has reinforced the idea
that the Oncotype DX assay is a predictor of chemotherapy response (Albain
2007). I believe it has a significant role in supporting the data we had from the
NSABP-B-20 study.
DR PAIK: The SWOG study Dr Kathy Albain presented has reinforced the idea
that the Oncotype DX assay is a predictor of chemotherapy response (Albain
2007). I believe it has a significant role in supporting the data we had from the
NSABP-B-20 study.
We compared our chemotherapy findings to the tamoxifen arm, which was used for the gene findings, so in one sense it was a highly biased population. It is reassuring to see a similar finding in node-positive disease, in which a high recurrence score from the Oncotype DX assay correlates with a higher degree of benefit from chemotherapy (Albain 2007; [4.2]).
The clinical utility of the Oncotype DX assay for patients with node-positive disease is still questionable. We need much more study because even the patient with a node-positive tumor determined to be at low risk by Oncotype DX profiling has a high baseline risk. Clinicians may have a hard time not administering chemotherapy to these patients, although biologically their expected benefit from it is minimal.
| Table of Contents | Top of Page |
INTERVIEWS
Neil Love, MD
Editor
Monica Morrow, MD
- Select publications
Ian E Smith, MD
- Select publications
Robert W Carlson, MD
- Select publications
Soonmyung Paik, MD
- Select publications
Breast Cancer Update
for Surgeons:
A CME Audio Series and Activity