
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: Can you comment on the recent paper published in The New
England Journal of Medicine that examined the benefit of paclitaxel in various
subgroups of patients, based on estrogen receptor (ER) and HER2 status?
DR LOVE: Can you comment on the recent paper published in The New
England Journal of Medicine that examined the benefit of paclitaxel in various
subgroups of patients, based on estrogen receptor (ER) and HER2 status?
![]() DR NORTON: Dan Hayes was the first author of this interesting paper
that retrospectively examined data from CALGB-9344, the trial that tested
dosing of adjuvant doxorubicin in combination with cyclophosphamide and
the addition of paclitaxel or not for women with node-positive breast cancer
(Hayes 2007).
DR NORTON: Dan Hayes was the first author of this interesting paper
that retrospectively examined data from CALGB-9344, the trial that tested
dosing of adjuvant doxorubicin in combination with cyclophosphamide and
the addition of paclitaxel or not for women with node-positive breast cancer
(Hayes 2007).
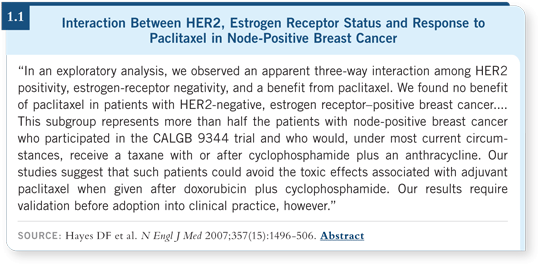
His team found that the benefits of paclitaxel were observed in all patients with HER2-overexpressed disease and in all patients with ER-negative disease. However, patients with HER2-nonoverexpressed disease and ER-positive disease did not seem to benefit from paclitaxel.
I’m a coauthor of that paper, and we stated clearly that these data are hypothesis generating. Any time you examine study data retrospectively, you can always find subsets in which all the difference is observed and differences that you do not observe in that subset. It’s critical that before we use these data to make decisions for individual patients, we examine other data sets and challenge the academic community, which has relevant data from other data sets.
Another issue complicating this analysis is defining HER2 positivity. We have a great deal of uncertainty in measuring HER2 because both IHC and FISH require an experienced pathologist and are dependent on the method of fixation and the handling of the tissue. We desperately need better means of finding out which cancers are HER2 driven so that we can properly understand the meaning of HER2 in this particular setting.
Even measurement of the estrogen receptor is something we haven’t determined with great authority, and methodological issues must be addressed. More critically, what does ER-positive mean? Is the cancer a luminal A type? Is it a luminal B type? We don’t necessarily know that by analyzing the estrogen receptor status, and subsets of these cases may be driving the whole observation.
An enormous amount of work has to be done in terms of understanding the fundamental biology of the cancer and, as a practicing clinician, I want to know these answers for my patients tomorrow, not in 10 years. We need to complete this work quickly, and we have to obtain the answers for practicing oncologists as fast as we can.
However, until we have those answers, the authors of the Hayes paper agree that it’s premature to deny paclitaxel to a patient with HER2-negative, ER-positive disease because we can’t say with certainty that these patients don’t benefit — simply that they didn’t seem to benefit in our retrospective analysis of our own data (1.1).
Track 6
![]() DR LOVE: Dennis Slamon feels that currently, anthracyclines do not
have a role in the adjuvant treatment of breast cancer. In clinical trials,
the incremental benefit of adjuvant anthracyclines is observed in patients
with HER2-positive, but not HER2-negative, disease, and in the BCIRG
006 trial, TCH was as beneficial as AC
DR LOVE: Dennis Slamon feels that currently, anthracyclines do not
have a role in the adjuvant treatment of breast cancer. In clinical trials,
the incremental benefit of adjuvant anthracyclines is observed in patients
with HER2-positive, but not HER2-negative, disease, and in the BCIRG
006 trial, TCH was as beneficial as AC![]() TH (Slamon 2006). What are
your thoughts on that?
TH (Slamon 2006). What are
your thoughts on that?
![]() DR NORTON: We all have extensive experience treating patients with anthracyclines
for metastatic breast cancer, and we know they’re active. I don’t know
why they would be active in Stage IV disease but not in early breast cancer.
This is something we need to examine carefully.
DR NORTON: We all have extensive experience treating patients with anthracyclines
for metastatic breast cancer, and we know they’re active. I don’t know
why they would be active in Stage IV disease but not in early breast cancer.
This is something we need to examine carefully.
For the sake of discussion, let’s assume there is a subset of patients who do not benefit from anthracyclines. How do we select those patients? The measurement of HER2 is not absolute at the present time. If you are using topoisomerase (TOPO) IIA as a manner of delineating who will benefit, the FISH probe for that is broad. You are not simply evaluating TOPO II but rather that whole region of the gene, and you do not know exactly what you are measuring.
In the CIRG experience, it was simply a subset of patients who were examined, and the studies are early in terms of the number of events, so you’re considering a subset of a subset (Press 2005). The results are provocative, but they must be examined carefully.
I believe the biochemical analysis of those specimens is a meritorious area of research, and it’s certainly hypothesis generating, but in terms of clinical extrapolation at this point, I don’t believe we’re ready to say to any patient that she will not benefit from anthracycline therapy.
![]() DR LOVE: A paper from MD Anderson published in the Journal of Clinical
Oncology reported a high risk of congestive heart failure among women aged
66 to 70 who received an anthracycline-based regimen in the adjuvant setting
(Pinder 2007; [1.2]). Can you comment on those data?
DR LOVE: A paper from MD Anderson published in the Journal of Clinical
Oncology reported a high risk of congestive heart failure among women aged
66 to 70 who received an anthracycline-based regimen in the adjuvant setting
(Pinder 2007; [1.2]). Can you comment on those data?
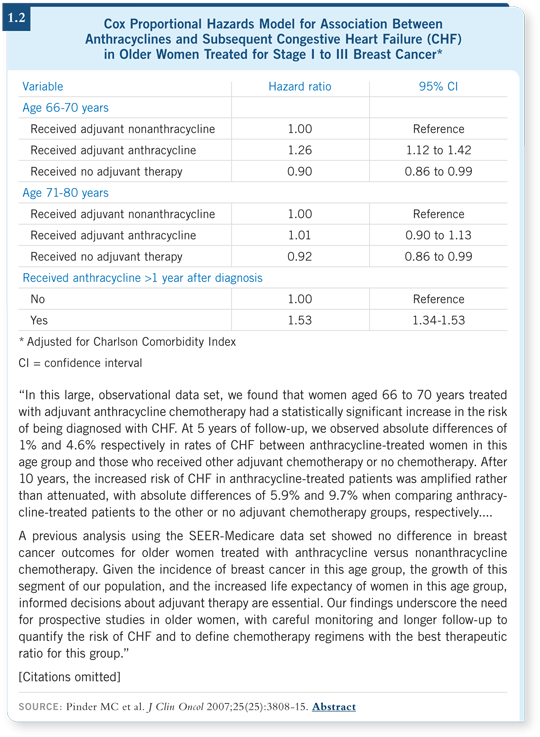
![]() DR NORTON: Remember, the patients receiving anthracyclines live longer, so
they will be at risk for basic cardiac problems longer, and that must be factored
in. The patients who relapse and die of metastatic disease we won’t see in the
long term to observe the cardiac toxicity.
DR NORTON: Remember, the patients receiving anthracyclines live longer, so
they will be at risk for basic cardiac problems longer, and that must be factored
in. The patients who relapse and die of metastatic disease we won’t see in the
long term to observe the cardiac toxicity.
Many aspects of this situation must be analyzed before we decide that regimens that have clearly been associated with the declining mortality from breast cancer — which is apparent in national SEER statistics — are not meritorious. We need to be careful about what we say and how we analyze and present the data.
Track 7
![]() DR LOVE: It’s my understanding that, within a few months, Oncotype DX assay results will include a quantitative ER measurement and that
they are working on HER2 also. Do you feel this strategy will affect
quality control of these measurements?
DR LOVE: It’s my understanding that, within a few months, Oncotype DX assay results will include a quantitative ER measurement and that
they are working on HER2 also. Do you feel this strategy will affect
quality control of these measurements?
![]() DR NORTON: I hope that everyone working on the biochemical analysis of
cancer will give a lot of attention to hormone-responsive genes. The fact that
the Oncotype DX assay can take a population of patients with ER-positive
disease, analyze a family of genes rather than ER alone and categorize them by
risk and whether they will benefit from adjuvant chemotherapy is extremely
instructive.
DR NORTON: I hope that everyone working on the biochemical analysis of
cancer will give a lot of attention to hormone-responsive genes. The fact that
the Oncotype DX assay can take a population of patients with ER-positive
disease, analyze a family of genes rather than ER alone and categorize them by
risk and whether they will benefit from adjuvant chemotherapy is extremely
instructive.
We are learning from the Oncotype DX assay, MammaPrint® and experimental assays that, although ER status correlates with the biochemical characteristics of the cancer, it doesn’t define the biochemical characteristics of the cancer, and we need to define those in a much more meaningful way. Quantitative ER results may be closer to defining the biochemical characteristics of the cancer.
Anyone who has used the Oncotype DX assay can tell you that cancer that appears to be hormone responsive and benign on the basis of IHC, when analyzed by Oncotype DX can surprise you and fall into a high-risk category that would benefit from chemotherapy.
Tracks 12-13
![]() DR LOVE: Can you provide an update on your work evaluating the dose
and schedule of capecitabine and combining it with bevacizumab?
DR LOVE: Can you provide an update on your work evaluating the dose
and schedule of capecitabine and combining it with bevacizumab?
![]() DR NORTON: Many of us have been using capecitabine for a long time
to treat metastatic breast cancer and are impressed with the activity of the
agent, but we are also impressed with the toxicity when administered for 14
days followed by a break for seven days. In many series and in many people’s
experience, up to one third of patients stop receiving this agent not because of
disease progression but because of toxicity, particularly because of hand-foot
syndrome.
DR NORTON: Many of us have been using capecitabine for a long time
to treat metastatic breast cancer and are impressed with the activity of the
agent, but we are also impressed with the toxicity when administered for 14
days followed by a break for seven days. In many series and in many people’s
experience, up to one third of patients stop receiving this agent not because of
disease progression but because of toxicity, particularly because of hand-foot
syndrome.
We initiated some animal experiments in which we examined the impact of capecitabine administered for 14 days on the perturbation of the tumor growth curves. We found that the maximum perturbation occurs at approximately a week of treatment and that the impact during the second week was dramatically reduced, but its toxicity was not.
Based on this information, we designed a regimen in a mouse experimental model evaluating seven days on and seven days off (Theodoulou 2007). We found we can increase the dose of capecitabine almost twofold compared to what we can deliver safely with a schedule of 14 days on, seven days off — and lo and behold, this resulted in increased tumor regression and more than doubled the survival benefit.
We then designed experimental models combining capecitabine with bevacizumab, and in the HER2-positive setting we also added trastuzumab, and these results have been published in abstract form (Traina 2007). The combination of all three drugs is profoundly effective, with not only significant inhibition of tumor growth or regression, but also improvement of survival of the animals with HER2-positive, ER-negative disease (1.3). The experiments with capecitabine and bevacizumab in the HER2-negative setting are still ongoing.
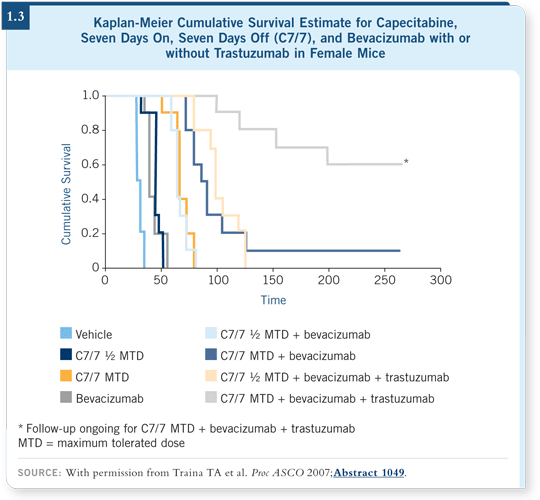
The important point to emphasize here is that achieving a proper dosing schedule of capecitabine enables us to combine it with biological agents and optimize the capecitabine effect without compromising the effect of the biological agent.
![]() DR LOVE: What did your dose-escalation study show?
DR LOVE: What did your dose-escalation study show?
![]() DR NORTON: We completed the Phase I-II trial and have determined that
a fixed dose of 2,000 milligrams BID for seven days is a well-tolerated
regimen (Theodoulou 2007). Indeed, many patients tolerated a higher dose
— 2,000 milligrams/2,500 milligrams in one day for seven days — and that
is delivering much more capecitabine than you can safely deliver on a 14-day
schedule. Even 1,000 mg/m2 for 14 days causes inordinate toxicity, for the
most part, and patients require dose modifications (Yap 2007).
DR NORTON: We completed the Phase I-II trial and have determined that
a fixed dose of 2,000 milligrams BID for seven days is a well-tolerated
regimen (Theodoulou 2007). Indeed, many patients tolerated a higher dose
— 2,000 milligrams/2,500 milligrams in one day for seven days — and that
is delivering much more capecitabine than you can safely deliver on a 14-day
schedule. Even 1,000 mg/m2 for 14 days causes inordinate toxicity, for the
most part, and patients require dose modifications (Yap 2007).
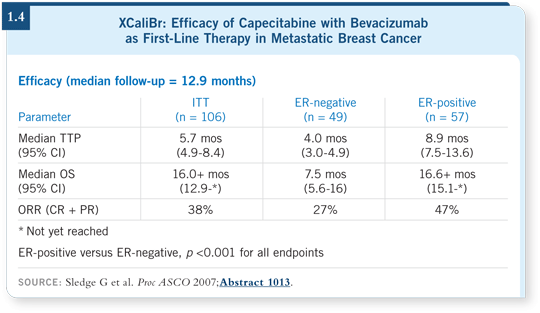
![]() DR LOVE: At ASCO 2007, George Sledge presented data from the XCaliBr
trial, which evaluated capecitabine with bevacizumab as front-line therapy for
metastatic breast cancer. What were your thoughts on the data (1.4)?
DR LOVE: At ASCO 2007, George Sledge presented data from the XCaliBr
trial, which evaluated capecitabine with bevacizumab as front-line therapy for
metastatic breast cancer. What were your thoughts on the data (1.4)?
![]() DR NORTON: Until we combine bevacizumab and capecitabine at the
optimum dose and schedule for capecitabine, we won’t know how effective that
combination is. In preclinical, experimental animal models, the combination is
effective, and I see no reason to believe that it won’t be effective in people.
DR NORTON: Until we combine bevacizumab and capecitabine at the
optimum dose and schedule for capecitabine, we won’t know how effective that
combination is. In preclinical, experimental animal models, the combination is
effective, and I see no reason to believe that it won’t be effective in people.
EDITOR'S NOTE
Another perspective on metastatic breast cancer
Neil Love, MD
- Select publications
INTERVIEWS
Larry Norton, MD
- Select publications
John Mackey, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Roundtable Discussions
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity