
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: What are some of the new trials being conducted by the
Cancer International Research Group?
DR LOVE: What are some of the new trials being conducted by the
Cancer International Research Group?
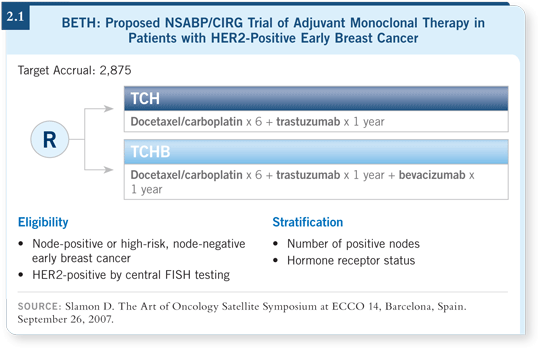
![]() DR MACKEY: The most exciting trial that we’re conducting is the BETH trial
(2.1), a collaborative effort between the NSABP and CIRG that is evaluating
bevacizumab in combination with trastuzumab in the adjuvant setting for
HER2-positive breast cancer. We proposed the idea because Dennis Slamon’s
laboratory found evidence of a profound synergistic interaction between those
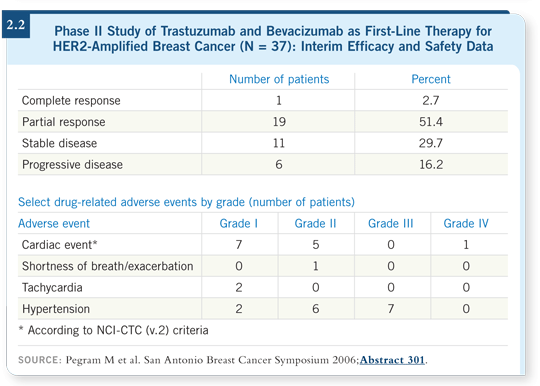
two drugs. In Phase I and then Phase II trials, they demonstrated tremendous efficacy with the two agents in advanced breast cancer (Pegram 2006; [2.2]). It
was only logical to move it into the adjuvant setting.
DR MACKEY: The most exciting trial that we’re conducting is the BETH trial
(2.1), a collaborative effort between the NSABP and CIRG that is evaluating
bevacizumab in combination with trastuzumab in the adjuvant setting for
HER2-positive breast cancer. We proposed the idea because Dennis Slamon’s
laboratory found evidence of a profound synergistic interaction between those
two drugs. In Phase I and then Phase II trials, they demonstrated tremendous efficacy with the two agents in advanced breast cancer (Pegram 2006; [2.2]). It
was only logical to move it into the adjuvant setting.
In the BETH trial design, we require that all tumors be submitted for central analysis prior to randomization to ensure true HER2 positivity. We will also examine a number of molecular markers, and we hope to tease out the subpopulation of patients who particularly benefit from the combination of trastuzumab and bevacizumab.
Tracks 2, 5
![]() DR LOVE: What do we know about the cardiac safety associated with
combining bevacizumab and trastuzumab?
DR LOVE: What do we know about the cardiac safety associated with
combining bevacizumab and trastuzumab?
![]() DR MACKEY: At present, we don’t have a lot of experience with bevacizumab
in combination with agents that have been associated with cardiotoxicity.
When I look at the literature, my take is that bevacizumab causes minimal
direct cardiotoxicity. It does have cardiovascular side effects. I believe a slight
increase occurs in bleeding and clotting, and a substantial proportion of
patients develop hypertension.
DR MACKEY: At present, we don’t have a lot of experience with bevacizumab
in combination with agents that have been associated with cardiotoxicity.
When I look at the literature, my take is that bevacizumab causes minimal
direct cardiotoxicity. It does have cardiovascular side effects. I believe a slight
increase occurs in bleeding and clotting, and a substantial proportion of
patients develop hypertension.
Trastuzumab, when used either with or after anthracyclines, clearly carries a cardiotoxicity signal. At present we have four years of follow-up on the adjuvant trastuzumab trials, so we don’t know whether the additional cardiotoxicity associated with trastuzumab following an anthracycline will be a big clinical problem in the future.
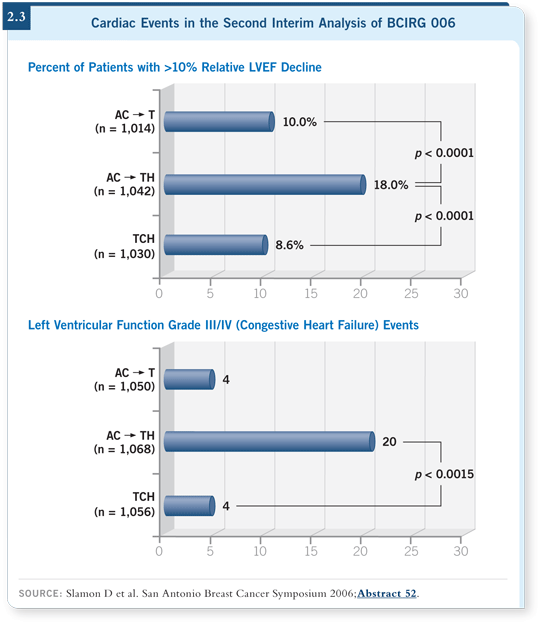
We’ve done some work in CIRG with the nonanthracycline adjuvant regimen of TCH (docetaxel, carboplatin and trastuzumab). Six cycles of TCH were administered in the adjuvant setting in BCIRG 006, and the rate of heart failure was low. Only four patients out of a thousand developed congestive heart failure (Slamon 2006; [2.3]). TCH appears to be an effective and fortunately noncardiotoxic adjuvant regimen, and we’re using it as the backbone for the BETH trial (2.1).
If you take a strictly scientific view, for a woman with early-stage breast
cancer that’s HER2 driven, the absolute benefits are statistically identical from
TCH and AC![]() TH. TCH is just as good in terms of efficacy. But a statistically
significant benefit is evident in terms of the safety profile of TCH
compared to an anthracycline/taxane backbone (Slamon 2006; [2.3]). When
I view the data, I say, “We have two treatments that are equivalent and one is
safer — end of discussion.”
TH. TCH is just as good in terms of efficacy. But a statistically
significant benefit is evident in terms of the safety profile of TCH
compared to an anthracycline/taxane backbone (Slamon 2006; [2.3]). When
I view the data, I say, “We have two treatments that are equivalent and one is
safer — end of discussion.”
![]() DR LOVE: In what situations, if any, are you using anthracyclines in adjuvant
therapy for women with HER2-positive breast cancer?
DR LOVE: In what situations, if any, are you using anthracyclines in adjuvant
therapy for women with HER2-positive breast cancer?
![]() DR MACKEY: Currently in my practice, I’m not.
DR MACKEY: Currently in my practice, I’m not.
Tracks 10-11
![]() DR LOVE: What do you think about the available data on the long-term
safety of anthracyclines?
DR LOVE: What do you think about the available data on the long-term
safety of anthracyclines?
![]() DR MACKEY: As breast cancer oncologists, we’ve been so concerned about curing the disease that we haven’t thoroughly evaluated the collateral damage. So we don’t have a lot of good long-term data on how people fare after adjuvant therapy with respect to cardiovascular morbidity. One of the best studies followed women treated with the MA5 regimen, which was an aggressive epirubicin combination. At five years, 25 percent of the women on the study had substantial drops in LVEF (Shepherd 2006).
DR MACKEY: As breast cancer oncologists, we’ve been so concerned about curing the disease that we haven’t thoroughly evaluated the collateral damage. So we don’t have a lot of good long-term data on how people fare after adjuvant therapy with respect to cardiovascular morbidity. One of the best studies followed women treated with the MA5 regimen, which was an aggressive epirubicin combination. At five years, 25 percent of the women on the study had substantial drops in LVEF (Shepherd 2006).
I believe one of the best data sets to address the issue comes from the SEER-Medicare database of women older than age 65 who received adjuvant therapy. Women who received CMF-like chemotherapy didn’t experience much incremental cardiotoxicity compared to age-matched controls, but among the women who received anthracyclines, an excess rate of CHF emerged (Pinder 2007; [1.2, page 12]). We’re concerned that a real problem exists, and it’s not well described because we haven’t been aware.
![]() DR LOVE: What are your thoughts about the hypothesis that anthracyclines have no role in the adjuvant treatment of breast cancer, regardless of HER2 status?
DR LOVE: What are your thoughts about the hypothesis that anthracyclines have no role in the adjuvant treatment of breast cancer, regardless of HER2 status?
![]() DR MACKEY: It has merit. If you consider all the trials comparing a nonanthracycline-
to an anthracycline-based adjuvant regimen, the incremental benefit of
the anthracyclines seems to be entirely confined to the population with HER2-positive disease. This was before adjuvant trastuzumab. The anthracyclines
seemed to be benefiting only the women with HER2-positive disease.
DR MACKEY: It has merit. If you consider all the trials comparing a nonanthracycline-
to an anthracycline-based adjuvant regimen, the incremental benefit of
the anthracyclines seems to be entirely confined to the population with HER2-positive disease. This was before adjuvant trastuzumab. The anthracyclines
seemed to be benefiting only the women with HER2-positive disease.
![]() DR LOVE: In the past, CIRG did a lot of work with adjuvant TAC, which
includes an anthracycline. In which situations are you using adjuvant anthracyclines
right now for HER2-negative disease?
DR LOVE: In the past, CIRG did a lot of work with adjuvant TAC, which
includes an anthracycline. In which situations are you using adjuvant anthracyclines
right now for HER2-negative disease?
![]() DR MACKEY: We compared TAC to a standard anthracycline regimen, FAC.
TAC provided a benefit in HER2-positive, HER2-negative, ER-positive
and ER-negative disease. There wasn’t a subgroup that didn’t benefit (Martin
2005). So we adopted TAC as our standard regimen here in Edmonton,
Alberta. Now we have to ask, do we need the anthracycline?
DR MACKEY: We compared TAC to a standard anthracycline regimen, FAC.
TAC provided a benefit in HER2-positive, HER2-negative, ER-positive
and ER-negative disease. There wasn’t a subgroup that didn’t benefit (Martin
2005). So we adopted TAC as our standard regimen here in Edmonton,
Alberta. Now we have to ask, do we need the anthracycline?
The strict scientific answer is that we don’t have prospective randomized trials to tell us whether you can drop the anthracycline in a regimen like TAC for a patient with HER2-negative disease. A trial is being launched, however, that is the brainchild of Steve Jones and US Oncology — it’s called the TC-TAC trial. The study will compare TAC to TC (docetaxel/cyclophosphamide) for patients with HER2-negative disease, with the intent to show that you can drop the anthracycline and obtain equivalent efficacy and less toxicity with TC.
Track 11
![]() DR LOVE: In your own practice outside of a clinical trial setting, how do
you handle decisions about whether to use an anthracycline for patients
with HER2-negative disease?
DR LOVE: In your own practice outside of a clinical trial setting, how do
you handle decisions about whether to use an anthracycline for patients
with HER2-negative disease?
![]() DR MACKEY: My preference and what I discuss with patients is that we proceed
with TC. I’m not administering anthracyclines to patients with HER2-negative
disease unless the patient is adamant that she wants one of the older regimens.
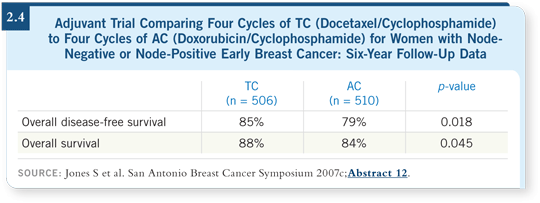
It’s clear that TC has achieved a survival advantage over AC ( Jones 2007c; [2.4]),
which is exciting because this population is unselected and includes patients
with HER2-positive disease who did not receive trastuzumab. So in a sense
this trial was stacked against the TC regimen, but TC is still outperforming AC in terms of safety and efficacy. With the survival advantage, I simply don’t see
where the anthracyclines fit into the treatment of HER2-negative disease.
DR MACKEY: My preference and what I discuss with patients is that we proceed
with TC. I’m not administering anthracyclines to patients with HER2-negative
disease unless the patient is adamant that she wants one of the older regimens.
It’s clear that TC has achieved a survival advantage over AC ( Jones 2007c; [2.4]),
which is exciting because this population is unselected and includes patients
with HER2-positive disease who did not receive trastuzumab. So in a sense
this trial was stacked against the TC regimen, but TC is still outperforming AC in terms of safety and efficacy. With the survival advantage, I simply don’t see
where the anthracyclines fit into the treatment of HER2-negative disease.
![]() DR LOVE: What if the patient had five positive nodes?
DR LOVE: What if the patient had five positive nodes?
![]() DR MACKEY: I would administer six cycles of TC.
DR MACKEY: I would administer six cycles of TC.
![]() DR LOVE: What about a new TCH using cyclophosphamide, instead of
carboplatin, with docetaxel and trastuzumab? US Oncology will evaluate this
regimen in an adjuvant clinical trial for patients with HER2-positive, early
breast cancer (2.5).
DR LOVE: What about a new TCH using cyclophosphamide, instead of
carboplatin, with docetaxel and trastuzumab? US Oncology will evaluate this
regimen in an adjuvant clinical trial for patients with HER2-positive, early
breast cancer (2.5).
![]() DR MACKEY: It’s a perfectly reasonable approach. TC is good chemotherapy,
and trastuzumab is good biological therapy. Whether it really matters what the
chemotherapy backbone is in terms of efficacy is not clear to me. However,
there is a lot to be said for the nonanthracycline backbone in terms of toxicity
(Slamon 2006; [2.3]).
DR MACKEY: It’s a perfectly reasonable approach. TC is good chemotherapy,
and trastuzumab is good biological therapy. Whether it really matters what the
chemotherapy backbone is in terms of efficacy is not clear to me. However,
there is a lot to be said for the nonanthracycline backbone in terms of toxicity
(Slamon 2006; [2.3]).

EDITOR'S NOTE
Another perspective on metastatic breast cancer
Neil Love, MD
- Select publications
INTERVIEWS
Larry Norton, MD
- Select publications
John Mackey, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Roundtable Discussions
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity