
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: What are some of the new areas of research that the NSABP is
considering?
DR LOVE: What are some of the new areas of research that the NSABP is
considering?
![]() DR GEYER: We have a trial in development designed to investigate therapies
for patients who have been through standard neoadjuvant chemotherapy
regimens but have residual disease at the time of surgery. We know from
the NSABP-B-27 study and others that, whereas the patients who achieve a
complete pathologic response (pCR) do well, those with residual disease don’t
do as well and are actually at high risk (Bear 2006; Mamounas 2005).
DR GEYER: We have a trial in development designed to investigate therapies
for patients who have been through standard neoadjuvant chemotherapy
regimens but have residual disease at the time of surgery. We know from
the NSABP-B-27 study and others that, whereas the patients who achieve a
complete pathologic response (pCR) do well, those with residual disease don’t
do as well and are actually at high risk (Bear 2006; Mamounas 2005).
Clearly some patients do have substantial residual disease after therapy. Their risk of recurrence is increased, and they don’t derive benefit from additional chemotherapy. So what we have proposed is a two-arm trial comparing a treatment with an oral VEGF inhibitor, sunitinib, to placebo to determine whether treating patients with “micrometastatic disease” with a therapy targeted specifically at VEGF with platelet-derived growth factor receptor (PDGFR) improves outcomes and lowers the risk for recurrence. Our plan is to administer the therapy for a year.
![]() DR LOVE: How do you think it will go taking patients out to a year on
sunitinib? From what I have seen in renal cell carcinoma (RCC), sunitinib is
not necessarily easy to tolerate.
DR LOVE: How do you think it will go taking patients out to a year on
sunitinib? From what I have seen in renal cell carcinoma (RCC), sunitinib is
not necessarily easy to tolerate.
![]() DR GEYER: That has been a concern, but these targeted therapies have been
administered for a year in initial trials, so we thought it would be reasonable
to plan for a year. We will learn if side effects are sufficient that patients
are discontinuing the medication. But our expectations are that patients will
complete the year of therapy.
DR GEYER: That has been a concern, but these targeted therapies have been
administered for a year in initial trials, so we thought it would be reasonable
to plan for a year. We will learn if side effects are sufficient that patients
are discontinuing the medication. But our expectations are that patients will
complete the year of therapy.
![]() DR LOVE: What is known about sunitinib as breast cancer treatment?
DR LOVE: What is known about sunitinib as breast cancer treatment?
![]() DR GEYER: The data that led to its approval in RCC indicate that sunitinib
effectively targets VEGF. Kathy Miller has evaluated it as a single agent for
patients with previously treated breast cancer, and the data showed a response
rate of 11 percent and an overall clinical benefit rate of 16 percent, which
is respectable for single-agent therapy in pretreated patient populations,
indicating that it does have activity in breast cancer (Miller 2005a).
DR GEYER: The data that led to its approval in RCC indicate that sunitinib
effectively targets VEGF. Kathy Miller has evaluated it as a single agent for
patients with previously treated breast cancer, and the data showed a response
rate of 11 percent and an overall clinical benefit rate of 16 percent, which
is respectable for single-agent therapy in pretreated patient populations,
indicating that it does have activity in breast cancer (Miller 2005a).
Track 3
![]() DR LOVE: What’s your take on the NSABP-B-40 neoadjuvant study with
bevacizumab and the ECOG-E5103 adjuvant study evaluating chemotherapy
with or without bevacizumab for patients with HER2-negative tumors?
DR LOVE: What’s your take on the NSABP-B-40 neoadjuvant study with
bevacizumab and the ECOG-E5103 adjuvant study evaluating chemotherapy
with or without bevacizumab for patients with HER2-negative tumors?
![]() DR GEYER: The results of the ECOG-E2100 trial in the metastatic setting
were compelling (Miller 2005b), and they justified moving bevacizumab into
adjuvant and neoadjuvant trials. I believe the ECOG adjuvant trial is a well
designed study that will answer a number of questions regarding whether
the addition of bevacizumab to chemotherapy improves outcomes and, if so,
whether duration is a critical component.
DR GEYER: The results of the ECOG-E2100 trial in the metastatic setting
were compelling (Miller 2005b), and they justified moving bevacizumab into
adjuvant and neoadjuvant trials. I believe the ECOG adjuvant trial is a well
designed study that will answer a number of questions regarding whether
the addition of bevacizumab to chemotherapy improves outcomes and, if so,
whether duration is a critical component.
NSABP-B-40 is one of the more complex trials that we have attempted to complete (3.1). Initially it was more of a pure chemotherapy trial investigating whether or not adding antimetabolites to sequential AC/docetaxel would improve pCR rates.
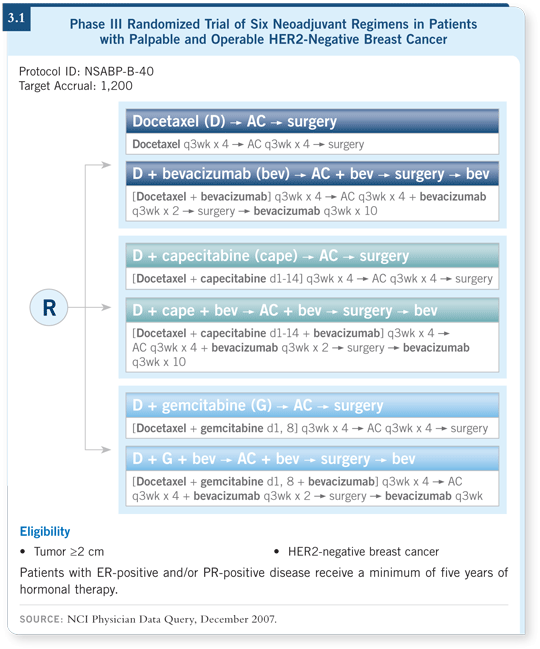
As the protocol was being developed, the data from the E2100 trial became available and a decision was made to ask a second question. So it is, in essence, a three-by-two study, in which half of the patients in each chemotherapy arm also receive bevacizumab and half of the patients do not.
The NSABP is also interested in collecting specimens from patients who do not achieve pCR to possibly understand evolving resistance mechanisms in addition to the up-front potential predictors for pCR. It’s a complicated and challenging study, and we believe it will be a gold mine of information when the study is finished.
Tracks 4-5
![]() DR LOVE: What is the current status of NSABP-B-42?
DR LOVE: What is the current status of NSABP-B-42?
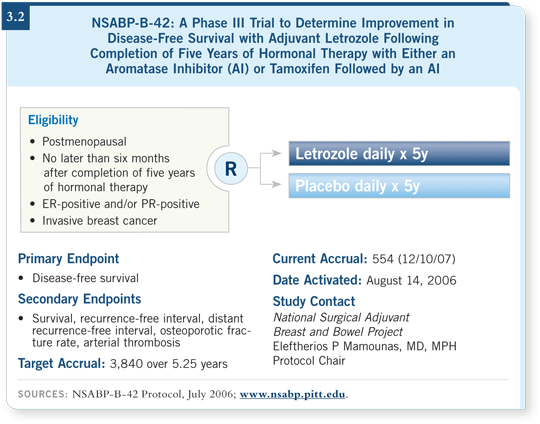
![]() DR GEYER: B-42 is a trial for postmenopausal women with hormone
receptor-positive breast cancer who have completed a standard five-year
duration of hormonal therapy either entirely consisting of an aromatase inhibitor
or up to three years of tamoxifen followed by an aromatase inhibitor (3.2).
The randomization is to either letrozole or placebo and the accrual to date is
at 489 out of a sample size of 3,840.
DR GEYER: B-42 is a trial for postmenopausal women with hormone
receptor-positive breast cancer who have completed a standard five-year
duration of hormonal therapy either entirely consisting of an aromatase inhibitor
or up to three years of tamoxifen followed by an aromatase inhibitor (3.2).
The randomization is to either letrozole or placebo and the accrual to date is
at 489 out of a sample size of 3,840.
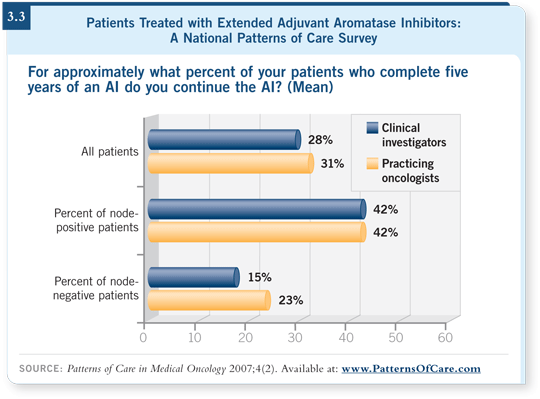
![]() DR LOVE: I know your first choice is to put a patient on the study, but if
that can’t be done, how do you approach the decision to stop or continue at
five years (3.3)?
DR LOVE: I know your first choice is to put a patient on the study, but if
that can’t be done, how do you approach the decision to stop or continue at
five years (3.3)?
![]() DR GEYER: We try to get some sense of the patients’ residual risk. We infer
that based on their baseline risk from their disease at presentation — a larger
tumor, a larger number of nodes and so on indicate a higher risk for recurrence
initially.
DR GEYER: We try to get some sense of the patients’ residual risk. We infer
that based on their baseline risk from their disease at presentation — a larger
tumor, a larger number of nodes and so on indicate a higher risk for recurrence
initially.
The assumption is that the relative reduction is fixed, so the residual risk beyond five years is higher. So if I have a patient with a large number of positive nodes, I will discuss with that patient the uncertainties regarding benefits of additional therapy, toxicities and so on. I believe that you also have to assess how the patient is tolerating the aromatase inhibitor.
Track 9
![]() DR LOVE: What are your thoughts about the BETH trial (2.1)?
DR LOVE: What are your thoughts about the BETH trial (2.1)?
![]() DR GEYER: The statistical design for BETH is chemotherapy with trastuzumab
with or without bevacizumab, and the protocol basically provides for two chemotherapy regimens. One is the TCH regimen that was used in the BCIRG 006 trial (Slamon 2006). The other regimen uses docetaxel at 100 mg/m2 every three weeks times three cycles followed by FEC with the epirubicin at 90 mg/m2. Patients on the docetaxel/FEC regimen receive the targeted therapy with the docetaxel. It is suspended during the FEC and then resumed after the FEC. Obviously the targeted therapy with TCH begins concurrently with the chemotherapy in both arms. All patients entered through the CIRG and NSABP will receive TC.
DR GEYER: The statistical design for BETH is chemotherapy with trastuzumab
with or without bevacizumab, and the protocol basically provides for two chemotherapy regimens. One is the TCH regimen that was used in the BCIRG 006 trial (Slamon 2006). The other regimen uses docetaxel at 100 mg/m2 every three weeks times three cycles followed by FEC with the epirubicin at 90 mg/m2. Patients on the docetaxel/FEC regimen receive the targeted therapy with the docetaxel. It is suspended during the FEC and then resumed after the FEC. Obviously the targeted therapy with TCH begins concurrently with the chemotherapy in both arms. All patients entered through the CIRG and NSABP will receive TC.
The idea of the two arms and the TCH justification arrive from the current results that Dr Slamon presented at the 2006 San Antonio meeting showing that outcomes with TCH versus AC![]() TH were statistically indistinguishable (3.4). The confidence intervals overlapped tremendously, and no statistically discernible difference in efficacy is apparent at this point, with a substantial number of events already reported.
TH were statistically indistinguishable (3.4). The confidence intervals overlapped tremendously, and no statistically discernible difference in efficacy is apparent at this point, with a substantial number of events already reported.
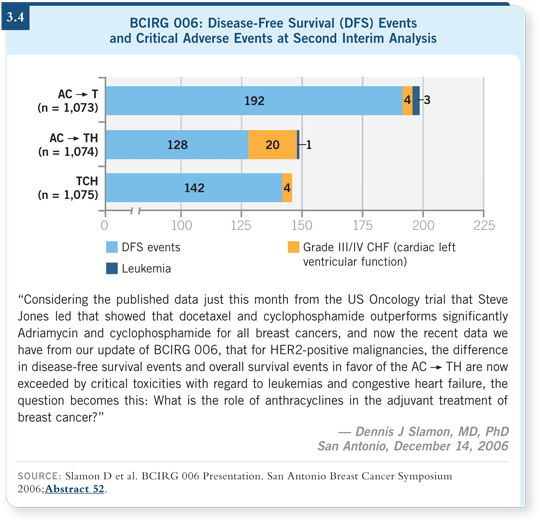
The other compelling part of the 006 trial relates to the cardiac toxicity issue. All the trials with trastuzumab following anthracyclines have shown a low tolerable rate of cardiac dysfunction, but clearly the lowest cardiotoxicity rates of any of the trials were seen on the TCH arm. So I believe the TCH is showing efficacy on a similar magnitude to the anthracycline and does have a more favorable safety profile.
Track 10
![]() DR LOVE: Let’s talk about the other major research strategy, embodied in the so-called ALTTO trial evaluating chemotherapy with trastuzumab, lapatinib or the combination of the two. Can you talk about the eligibility and design of that study?
DR LOVE: Let’s talk about the other major research strategy, embodied in the so-called ALTTO trial evaluating chemotherapy with trastuzumab, lapatinib or the combination of the two. Can you talk about the eligibility and design of that study?
![]() DR GEYER: ALTTO is a particularly large trial of adjuvant or neoadjuvant
targeted treatment for women with HER2-positive, operable breast cancer. Investigators can choose from a number of recommended anthracycline
regimens, and if a patient’s ejection fraction is 50 percent or higher, that patient can enter the trial and be randomly assigned to one of four targeted therapy options.
DR GEYER: ALTTO is a particularly large trial of adjuvant or neoadjuvant
targeted treatment for women with HER2-positive, operable breast cancer. Investigators can choose from a number of recommended anthracycline
regimens, and if a patient’s ejection fraction is 50 percent or higher, that patient can enter the trial and be randomly assigned to one of four targeted therapy options.
![]() DR LOVE: What clinical research information do we have on the combination of trastuzumab and lapatinib?
DR LOVE: What clinical research information do we have on the combination of trastuzumab and lapatinib?
![]() DR GEYER: A Phase I dose-finding study showed that fatigue is the dose-limiting toxicity. With the standard dose of trastuzumab, the lapatinib has to be reduced to 1,000 mg/day from 1,500 mg/day. So the lapatinib dose must be adjusted according to the partner with which you’re administering it.
DR GEYER: A Phase I dose-finding study showed that fatigue is the dose-limiting toxicity. With the standard dose of trastuzumab, the lapatinib has to be reduced to 1,000 mg/day from 1,500 mg/day. So the lapatinib dose must be adjusted according to the partner with which you’re administering it.
Track 12
![]() DR LOVE: Can you review what we know from the clinical studies of lapatinib?
DR LOVE: Can you review what we know from the clinical studies of lapatinib?
![]() DR GEYER: A study that I was involved with, the lapatinib/capecitabine versus capecitabine alone study, crossed an early reporting boundary on the first interim analysis and so had accrual closed early (Geyer 2006). The data that we presented at the last ASCO meeting were an update of the efficacy data. That represents about four and a half additional months to what was in the manuscript in The New England Journal of Medicine (Geyer 2006). So the numbers have changed a little, but the overall findings are the same.
DR GEYER: A study that I was involved with, the lapatinib/capecitabine versus capecitabine alone study, crossed an early reporting boundary on the first interim analysis and so had accrual closed early (Geyer 2006). The data that we presented at the last ASCO meeting were an update of the efficacy data. That represents about four and a half additional months to what was in the manuscript in The New England Journal of Medicine (Geyer 2006). So the numbers have changed a little, but the overall findings are the same.
The hazard ratio for time to progression was 0.57, and the median time to progression was 4.3 for capecitabine to 6.2 months for the combination, so the data held for the initial publication.
![]() DR LOVE: What did you observe in terms of side effects and toxicity in that study?
DR LOVE: What did you observe in terms of side effects and toxicity in that study?
![]() DR GEYER: The important thing to remember about the trial is that the comparator arm was capecitabine at 2,500 mg/m2, and that is a dose that most practicing oncologists no longer use to begin therapy. I believe most of us are starting at 2,000 mg/m2 or perhaps a little less. The only significant difference between the toxicity of the lapatinib/capecitabine and that dose of capecitabine was an increase in the rate of diarrhea from 40 percent to 60 percent, but the bulk of that increase was in the Grade I/Grade II range. So this dose did not substantially increase the toxicity that we see with 2,500 mg/m2.
DR GEYER: The important thing to remember about the trial is that the comparator arm was capecitabine at 2,500 mg/m2, and that is a dose that most practicing oncologists no longer use to begin therapy. I believe most of us are starting at 2,000 mg/m2 or perhaps a little less. The only significant difference between the toxicity of the lapatinib/capecitabine and that dose of capecitabine was an increase in the rate of diarrhea from 40 percent to 60 percent, but the bulk of that increase was in the Grade I/Grade II range. So this dose did not substantially increase the toxicity that we see with 2,500 mg/m2.
About the same number of patients — approximately 13 percent — discontinued therapy due to side effects on both arms. Overall, it was impressive that lapatinib didn’t notably increase the toxicity, but this is a regimen that must be monitored and doses modified, or patients can experience substantial toxicity.
Track 17
![]() DR LOVE: Can you discuss the NSABP-B-41 neoadjuvant trial (3.5)?
DR LOVE: Can you discuss the NSABP-B-41 neoadjuvant trial (3.5)?
![]() DR GEYER: In this neoadjuvant trial, we use AC followed by weekly paclitaxel
along with trastuzumab or lapatinib or the combination using pCR as a primary endpoint. After surgery, everyone completes the year of targeted therapy with trastuzumab.
DR GEYER: In this neoadjuvant trial, we use AC followed by weekly paclitaxel
along with trastuzumab or lapatinib or the combination using pCR as a primary endpoint. After surgery, everyone completes the year of targeted therapy with trastuzumab.
Lapatinib clearly has a different mechanism of action than trastuzumab. My hope is that patients responding to trastuzumab will be different from patients responding to lapatinib and to the combination of the two. I believe that it is important to see these trials completed, to collect the tissue and to find out whether there are fundamental differences among these women that can be identified before we begin therapy.
Then we can choose the correct drug from day one. That is why I believe the neoadjuvant studies are of particular importance here — to provide us with that kind of information.

EDITOR'S NOTE
Another perspective on metastatic breast cancer
Neil Love, MD
- Select publications
INTERVIEWS
Larry Norton, MD
- Select publications
John Mackey, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Roundtable Discussions
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity