
 |
||||||||

| Tracks 1-25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Discussion
Tracks 1-3

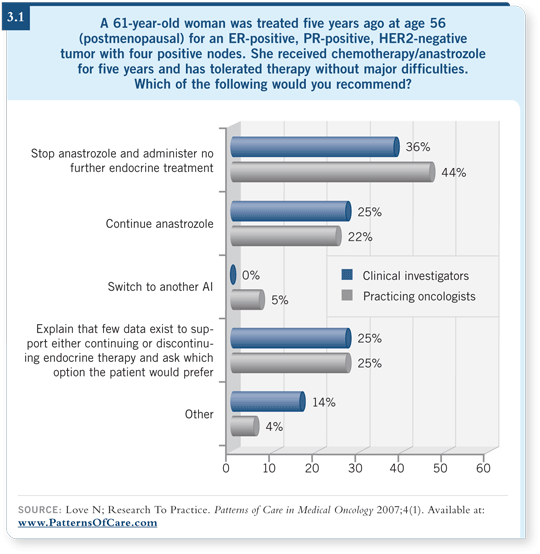
![]() DR LOVE: What are you generally doing for patients who complete five
years of an adjuvant aromatase inhibitor (3.1)?
DR LOVE: What are you generally doing for patients who complete five
years of an adjuvant aromatase inhibitor (3.1)?
![]() DR CHLEBOWSKI: At present, when I start patients on an aromatase inhibitor,
I tell them about the lack of data, the potential safety concerns and my plan to
prescribe the aromatase inhibitor for at least seven years.
DR CHLEBOWSKI: At present, when I start patients on an aromatase inhibitor,
I tell them about the lack of data, the potential safety concerns and my plan to
prescribe the aromatase inhibitor for at least seven years.
I also say we might have more data on duration as time goes on. I do that up front because a fair number of patients who complete five years of therapy say, “I’m done with my cancer treatment.”
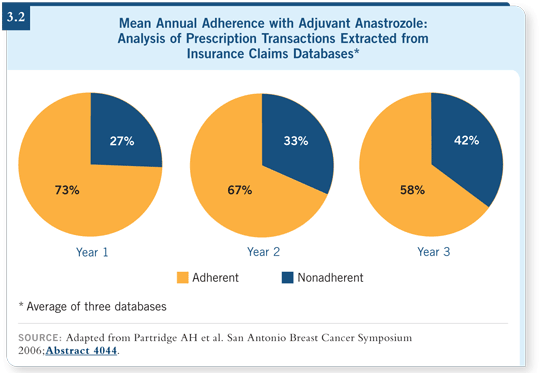
Of course, this gets back to the issue of adherence with these longer regimens. I recently conducted a review evaluating tamoxifen adherence in three population- based studies (Chlebowski 2006).
It appears that 30 to 50 percent of women are stopping their tamoxifen treatment between the fourth and fifth year, and Dr Partridge presented a study demonstrating the same results with aromatase inhibitors (Partridge 2006; [3.2]).
Not many oncologists believe that up to half of the women aren’t taking their medication in the fourth year. It’s interesting to talk about longer duration of therapy if half of the women are taking a shorter duration volitionally.
![]() DR LOVE: MJ, how do you approach the patient who has completed five years
of adjuvant hormonal therapy?
DR LOVE: MJ, how do you approach the patient who has completed five years
of adjuvant hormonal therapy?
![]() DR JAHANZEB: After five years of tamoxifen, it’s easy. I fall back on the
MA17 data and offer an aromatase inhibitor (Goss 2005).
DR JAHANZEB: After five years of tamoxifen, it’s easy. I fall back on the
MA17 data and offer an aromatase inhibitor (Goss 2005).
After five years of an aromatase inhibitor, I’m left with telling the patient that the risk of relapse continues and something ought to be done, but I don’t know what that something is.
If they are tolerating the aromatase inhibitor, are able to afford it and want to continue taking it, like Rowan, I say, “Continue to take it .” I’m not, however, telling them to take it two more years because I don’t know the optimal duration. I’m hoping that within two years, more snippets of data will emerge, and then I’ll update the recommendation.
Without much data but with some gut feeling, I am more emphatic about asking those patients with strongly ER-positive/ PR-positive disease to continue taking it. With those who have weakly ER-positive disease, I’m not that particular about it.
![]() DR DICKLER: I often opt for continuing therapy, particularly for my patients
at high risk.
DR DICKLER: I often opt for continuing therapy, particularly for my patients
at high risk.
If we know letrozole extends the benefits of five years of tamoxifen and the patient is at the five-year junction, I want to protect the patient during years five through possibly 10 after finishing five years of an aromatase inhibitor.
For this patient who had positive nodes, I would offer her continued aromatase inhibitor therapy, watch her bone density closely and take it on a year-by-year basis. I also wonder whether switching to tamoxifen would be the optimal strategy. I’m anxiously awaiting the results from BIG 1-98 in terms of the sequencing strategy. Switching after several years from one mechanism of action to another may be the best approach.
![]() DR CHLEBOWSKI: Some people will recommend starting tamoxifen first so
they can administer seven years of therapy. I don’t find that attractive because
we have the same lack of data for that strategy as we do for seven years of an
aromatase inhibitor, and you’re spotting people the first couple years of recurrences.
You don’t need to administer tamoxifen first to administer more than
five years of aromatase inhibitor therapy, as I view the evidence.
DR CHLEBOWSKI: Some people will recommend starting tamoxifen first so
they can administer seven years of therapy. I don’t find that attractive because
we have the same lack of data for that strategy as we do for seven years of an
aromatase inhibitor, and you’re spotting people the first couple years of recurrences.
You don’t need to administer tamoxifen first to administer more than
five years of aromatase inhibitor therapy, as I view the evidence.
Track 4-8

![]() DR LOVE: Maura, this is a difficult situation — 10 positive nodes and a history of congestive heart failure. What do you think you would have done?
DR LOVE: Maura, this is a difficult situation — 10 positive nodes and a history of congestive heart failure. What do you think you would have done?
![]() DR DICKLER: It’s a difficult case because she’s not that old, but she has significant comorbidity from cardiovascular disease and breast cancer. These cases are a little easier when the patient is sitting in front of you because seeing what she looks like and getting her take on treatment is important.
DR DICKLER: It’s a difficult case because she’s not that old, but she has significant comorbidity from cardiovascular disease and breast cancer. These cases are a little easier when the patient is sitting in front of you because seeing what she looks like and getting her take on treatment is important.
The use of an anthracycline is not absolutely contraindicated in this setting, but this is a good case in which to consider the use of docetaxel and cyclophosphamide (TC). It’s short therapy, and it’s safer than AC. I would talk to her about dose-dense AC![]() T and also TC.
T and also TC.
For patients who are particularly risk averse and for whom I want to use chemotherapy, I even still bring CMF into the mix. It is better tolerated in general, and I can probably get her through it. I would talk with her about each regimen and the potential benefits.
![]() DR LOVE: Would you consider using an anthracycline for this patient?
DR LOVE: Would you consider using an anthracycline for this patient?
![]() DR DICKLER: She has an ejection fraction of 51 percent. You can follow her
closely and obtain a MUGA scan after two cycles. It’s her other comorbidities
that worry me equally.
DR DICKLER: She has an ejection fraction of 51 percent. You can follow her
closely and obtain a MUGA scan after two cycles. It’s her other comorbidities
that worry me equally.
AC can be a tough regimen. We use steroids as antiemetics, and she’s a diabetic, although with TC we do the same. That’s why I’d bring CMF into the mix because it’s less stressful and you don’t need as many steroids in terms of the antiemetics.
I wouldn’t rule out an anthracycline because 10 positive nodes pose a significant risk. This is a patient for whom I would conduct an evidence-of-disease evaluation.
I would do a CT scan and a bone scan because if I found any evidence of distant metastatic disease, I would offer her hormonal therapy. It would change her treatment significantly, and I could save her the morbidity of chemotherapy.
![]() DR CHLEBOWSKI: I like the concept of TC, and we’ve been using it. I might
use six instead of four cycles and tell her we have no data.
DR CHLEBOWSKI: I like the concept of TC, and we’ve been using it. I might
use six instead of four cycles and tell her we have no data.
![]() DR LOVE: What happened with this patient?
DR LOVE: What happened with this patient?
![]() DR JAHANZEB: We talked to her about CMF, and we felt that most oncologists
in the United States wouldn’t consider it adequate therapy for this type of
situation. We also talked about TC and the less-often-used regimen of CMF
followed by docetaxel for three cycles.
DR JAHANZEB: We talked to her about CMF, and we felt that most oncologists
in the United States wouldn’t consider it adequate therapy for this type of
situation. We also talked about TC and the less-often-used regimen of CMF
followed by docetaxel for three cycles.
We ultimately decided on TC, and without any data, we told her we believed four cycles were not enough and that maybe she should receive six cycles. She was treated with five cycles and had difficulty.
She received steroids, which made the diabetes management difficult with every cycle. She also developed progressive fatigue.
We told her, “We can’t tell you whether there’s a difference between five and six cycles. If this is how you feel, then let’s call it a day and send you for radiation therapy,” which is what we did.
![]() DR LOVE: Are you observing that TC is better tolerated than AC?
DR LOVE: Are you observing that TC is better tolerated than AC?
![]() DR CHLEBOWSKI: It’s about the same — maybe a little better. I don’t experience
too much difficulty with it. Taxanes can be difficult for anybody,
especially docetaxel in terms of taking it serially, but we’ve been pleased with
our ability to deliver TC.
DR CHLEBOWSKI: It’s about the same — maybe a little better. I don’t experience
too much difficulty with it. Taxanes can be difficult for anybody,
especially docetaxel in terms of taking it serially, but we’ve been pleased with
our ability to deliver TC.
![]() DR JAHANZEB: I find that single-agent docetaxel at 100 mg/m2 is more difficult
to tolerate than TC, which uses docetaxel at 75 mg/m2.
DR JAHANZEB: I find that single-agent docetaxel at 100 mg/m2 is more difficult
to tolerate than TC, which uses docetaxel at 75 mg/m2.
![]() DR DICKLER: I agree. I’ve had some good experiences with TC. I believe it’s
better tolerated, in general, than AC.
DR DICKLER: I agree. I’ve had some good experiences with TC. I believe it’s
better tolerated, in general, than AC.
When I have a patient with higher-risk, node-negative disease, with whom I
sometimes discuss AC![]() T but I don’t feel as strongly, I say I have a middle-of-the-road regimen — TC — which is associated with less cardiac toxicity.
Patients are comforted by that.
T but I don’t feel as strongly, I say I have a middle-of-the-road regimen — TC — which is associated with less cardiac toxicity.
Patients are comforted by that.
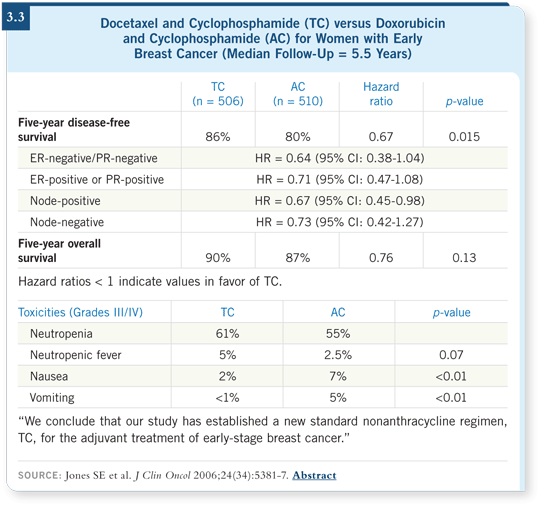
I don’t use much AC for four cycles anymore. If TC offers an improved disease-free survival and none of the risk for congestive heart failure (Jones 2006; [3.3]), my bias is to use TC instead of AC.
Tracks 9-13
![]() DR CHLEBOWSKI: This patient has a whole range of treatment options, and
this is one setting in which I’m almost reactive with the patient. In a clinical
setting, you can go from the minimalist approach with tamoxifen to saying,
“I’m going to use combination chemotherapy and try to get a higher response
rate. Then maybe I’ll switch to hormone therapy.”
DR CHLEBOWSKI: This patient has a whole range of treatment options, and
this is one setting in which I’m almost reactive with the patient. In a clinical
setting, you can go from the minimalist approach with tamoxifen to saying,
“I’m going to use combination chemotherapy and try to get a higher response
rate. Then maybe I’ll switch to hormone therapy.”
The tricky part with a younger patient is trying to explain the business at hand. It’s important to figure out how this person views her life with this condition. Does she want to aggressively attack it? Some of us would feel strongly that we want to use chemotherapy to reduce the tumor burden and then do something else.
Or you can consider that the first regimen you start somebody off with — this is more my guiding principle — will be the one they will be on longest. In that case, you could either use tamoxifen or capecitabine as the initial therapy. I believe somebody at this age is likely to say, “I want to be as aggressive as possible.” Then I go with a combination chemotherapy regimen, and we’ve been using capecitabine and docetaxel.
![]() DR LOVE: MJ, how would you think this through?
DR LOVE: MJ, how would you think this through?
![]() DR JAHANZEB: I like to get a handle on the velocity of the disease, which
is critical. Because of our biases, patients who are younger, have a larger
tumor burden or have rapidly progressing disease tend to receive combination
chemotherapy. Even if the disease is ER-positive or PR-positive, we start with
chemotherapy, which is appropriate, if they have visceral crisis.
DR JAHANZEB: I like to get a handle on the velocity of the disease, which
is critical. Because of our biases, patients who are younger, have a larger
tumor burden or have rapidly progressing disease tend to receive combination
chemotherapy. Even if the disease is ER-positive or PR-positive, we start with
chemotherapy, which is appropriate, if they have visceral crisis.
In this case, although the metastases are large, you know that she has not had her breasts for about four years. So it took at least four years for this to develop in her liver. That would tell me I could gamble on the side of being conservative and first try tamoxifen. My philosophy in metastatic disease is usually to do as little as possible for as long as possible.
So I would start with tamoxifen, keeping a close eye and evaluating her sooner rather than later for rapid progression. I wouldn’t expect tamoxifen to work before an average of four months, but this type of patient I would reevaluate within eight weeks to confirm that she’s not rapidly progressing.
![]() DR LOVE: Let’s say she responds well to tamoxifen, and then her disease
progresses. Then what?
DR LOVE: Let’s say she responds well to tamoxifen, and then her disease
progresses. Then what?
![]() DR JAHANZEB: At that time, I would use ovarian suppression with an aromatase
inhibitor. Then, in the third-line setting, one could consider fulvestrant
with ovarian suppression. I would like to maximize all the benefit from
hormonal therapy and then switch to sequential single-agent chemotherapy. I
like to start with nonalopecia-producing and less toxic single agents first, then
go to the alopecia-producing or more toxic single agents and then to combination
chemotherapy.
DR JAHANZEB: At that time, I would use ovarian suppression with an aromatase
inhibitor. Then, in the third-line setting, one could consider fulvestrant
with ovarian suppression. I would like to maximize all the benefit from
hormonal therapy and then switch to sequential single-agent chemotherapy. I
like to start with nonalopecia-producing and less toxic single agents first, then
go to the alopecia-producing or more toxic single agents and then to combination
chemotherapy.
![]() DR LOVE: What happened with this patient?
DR LOVE: What happened with this patient?
![]() DR DICKLER: She obtained various opinions reflecting the options at hand.
She opted to receive combination chemotherapy and was treated with doxorubicin
and docetaxel for six cycles. She had a tremendous response initially.
DR DICKLER: She obtained various opinions reflecting the options at hand.
She opted to receive combination chemotherapy and was treated with doxorubicin
and docetaxel for six cycles. She had a tremendous response initially.
After six cycles of chemotherapy, she received leuprolide and tamoxifen. She had a short-lived response to hormonal therapy, and within several weeks we diagnosed progression of disease in her liver.
![]() DR LOVE: Rowan, if you saw this patient for a second opinion after she had
a great response to chemotherapy and not much of a response to tamoxifen,
what would you recommend?
DR LOVE: Rowan, if you saw this patient for a second opinion after she had
a great response to chemotherapy and not much of a response to tamoxifen,
what would you recommend?
![]() DR CHLEBOWSKI: I don’t believe we have many data indicating that how
quickly somebody’s disease progresses on hormone therapy determines
whether she will respond to further hormone therapy. From the limited
data from randomized trials, 30 to 40 percent of patients derive a clinical
benefit from a second hormonal agent. Having said that, if she hasn’t received
capecitabine before, I would probably use that.
DR CHLEBOWSKI: I don’t believe we have many data indicating that how
quickly somebody’s disease progresses on hormone therapy determines
whether she will respond to further hormone therapy. From the limited
data from randomized trials, 30 to 40 percent of patients derive a clinical
benefit from a second hormonal agent. Having said that, if she hasn’t received
capecitabine before, I would probably use that.
![]() DR LOVE: What about capecitabine and bevacizumab?
DR LOVE: What about capecitabine and bevacizumab?
![]() DR CHLEBOWSKI: That combination is reasonable and could be considered.
DR CHLEBOWSKI: That combination is reasonable and could be considered.
![]() DR JAHANZEB: Assuming we know she received docetaxel previously, I
would be a little leery about using capecitabine/bevacizumab because that
particular trial was negative. She received both an anthracycline and a taxane,
and those patients did not seem to benefit in Kathy Miller’s study of bevacizumab
with capecitabine (Miller 2005). At the point of progression —
knowing that she had received an anthracycline and a taxane — I would have
tried capecitabine alone.
DR JAHANZEB: Assuming we know she received docetaxel previously, I
would be a little leery about using capecitabine/bevacizumab because that
particular trial was negative. She received both an anthracycline and a taxane,
and those patients did not seem to benefit in Kathy Miller’s study of bevacizumab
with capecitabine (Miller 2005). At the point of progression —
knowing that she had received an anthracycline and a taxane — I would have
tried capecitabine alone.
![]() DR LOVE: Maura, what happened?
DR LOVE: Maura, what happened?
![]() DR DICKLER: I presented this case because I have learned so much from this
patient over many years. She was offered participation in that “negative” trial
of capecitabine with or without bevacizumab. She was assigned to receive
capecitabine and bevacizumab at the end of 2001.
DR DICKLER: I presented this case because I have learned so much from this
patient over many years. She was offered participation in that “negative” trial
of capecitabine with or without bevacizumab. She was assigned to receive
capecitabine and bevacizumab at the end of 2001.
It’s now 2007, and she’s still on that therapy. She had a partial response in terms of the scans, in that her liver never completely normalized, but recently she had a PET scan and no uptake was observed in the liver.
She has always had significant hand-foot syndrome and has undergone numerous dose reductions. About two years ago, she also developed significant hypertension and proteinuria that ranged from 1.0 to 3.5 grams. She has never had nephrotic syndrome but has had changes in her lipid profile.
The hypertension is managed with three medications — beta blockers, ACE inhibitors and calcium channel blockers. For the proteinuria, she has been closely followed by one of our nephrologists. We’ve managed it, at times, by arranging a break from the bevacizumab, which reduces the proteinuria relatively quickly. In a couple of weeks, we have been able to reduce the proteinuria to about one gram. Also, at times, we’ve reduced her dose of bevacizumab.
More recently, she’s developed some chest heaviness that’s been extensively worked up. Although we’re unsure of the etiology, she believes it’s the capecitabine. Because we have no definite evidence of disease and I believe she’s deriving a lot of toxicity from the capecitabine, I recently offered her letrozole and stopped the capecitabine.
![]() DR LOVE: What about the bevacizumab?
DR LOVE: What about the bevacizumab?
![]() DR DICKLER: We are running a feasibility study of letrozole with bevacizumab,
in which we’ve enrolled 43 patients. Although she’s not part of that trial, it’s
proved safe, and we are in the late phases of designing a randomized trial in the
CALGB evaluating endocrine therapy with or without bevacizumab, so I will
probably continue the bevacizumab but closely watch her proteinuria.
DR DICKLER: We are running a feasibility study of letrozole with bevacizumab,
in which we’ve enrolled 43 patients. Although she’s not part of that trial, it’s
proved safe, and we are in the late phases of designing a randomized trial in the
CALGB evaluating endocrine therapy with or without bevacizumab, so I will
probably continue the bevacizumab but closely watch her proteinuria.
Tracks 14-16
![]() DR LOVE: MJ, how would you have thought through the decision to
switch this patient from tamoxifen to an aromatase inhibitor?
DR LOVE: MJ, how would you have thought through the decision to
switch this patient from tamoxifen to an aromatase inhibitor?
![]() DR JAHANZEB: I would have obtained a hormone profile to assess her
menopausal status. I’ve had patients who three years after chemotherapy have
resumed their ovarian function, which scares me. So not only would I have
obtained one, I would have repeated it six months later.
DR JAHANZEB: I would have obtained a hormone profile to assess her
menopausal status. I’ve had patients who three years after chemotherapy have
resumed their ovarian function, which scares me. So not only would I have
obtained one, I would have repeated it six months later.
If she was menopausal, I would have discussed switching to an aromatase inhibitor, but I would try to talk her into staying on tamoxifen for five years and then taking an aromatase inhibitor for five years.
![]() DR DICKLER: I feel similarly cautious about sequencing therapies for these
patients too early because I have found that ovarian function can resume
later than you expect. I suspect this woman is menopausal, but I would have
checked estradiol levels frequently over time and then waited longer. However,
you could probably sequence her therapies at this juncture.
DR DICKLER: I feel similarly cautious about sequencing therapies for these
patients too early because I have found that ovarian function can resume
later than you expect. I suspect this woman is menopausal, but I would have
checked estradiol levels frequently over time and then waited longer. However,
you could probably sequence her therapies at this juncture.
Following TAC for six cycles, most patients become amenorrheic. I’d talk to her about it, but I wouldn’t want to switch her too early to an aromatase inhibitor, which wouldn’t be effective if she still had some residual ovarian function.
![]() DR CHLEBOWSKI: I don’t check hormone levels because I wouldn’t consider
switching her regardless of her hormone levels. If you obtain an estradiol level
today, it will not tell you what will happen next week or the week after. For a
patient who is sexually active, menstruating and taking an aromatase inhibitor,
which is an egg stimulant, you run the risk of multiple pregnancies in addition
to using ineffective therapy.
DR CHLEBOWSKI: I don’t check hormone levels because I wouldn’t consider
switching her regardless of her hormone levels. If you obtain an estradiol level
today, it will not tell you what will happen next week or the week after. For a
patient who is sexually active, menstruating and taking an aromatase inhibitor,
which is an egg stimulant, you run the risk of multiple pregnancies in addition
to using ineffective therapy.
Many patients are probably being switched early, and the consequences are unclear. This is about the only group with which I’m enthusiastic about using tamoxifen for a while.
![]() DR LOVE: What about ovarian suppression?
DR LOVE: What about ovarian suppression?
![]() DR CHLEBOWSKI: Although Phase II trials are being conducted, and Bob
Carlson is running one with about 50 patients (Carlson 2007), it’s not clear how
well or completely you’re suppressing ovarian function with goserelin. I wouldn’t
be willing to use goserelin in the adjuvant setting with an aromatase inhibitor.
DR CHLEBOWSKI: Although Phase II trials are being conducted, and Bob
Carlson is running one with about 50 patients (Carlson 2007), it’s not clear how
well or completely you’re suppressing ovarian function with goserelin. I wouldn’t
be willing to use goserelin in the adjuvant setting with an aromatase inhibitor.
Track 17-20

![]() DR DICKLER: If this patient came to us, I would offer her participation in our
pilot feasibility trial evaluating dose-dense AC
DR DICKLER: If this patient came to us, I would offer her participation in our
pilot feasibility trial evaluating dose-dense AC![]() nab paclitaxel with bevacizumab.
Otherwise, I would use dose-dense AC
nab paclitaxel with bevacizumab.
Otherwise, I would use dose-dense AC![]() T.
T.
![]() DR CHLEBOWSKI: We would probably administer six cycles of TAC.
DR CHLEBOWSKI: We would probably administer six cycles of TAC.
![]() DR LOVE: What did you end up doing for this patient?
DR LOVE: What did you end up doing for this patient?
![]() DR JAHANZEB: We offered her participation in a study of metronomic chemotherapy, which she declined. She clearly had high-risk disease, despite
negative nodes. So we discussed our usual high-risk regimens — TAC,
AC
DR JAHANZEB: We offered her participation in a study of metronomic chemotherapy, which she declined. She clearly had high-risk disease, despite
negative nodes. So we discussed our usual high-risk regimens — TAC,
AC![]() docetaxel or dose-dense therapy — in that order, with de-emphasis on
the dose-dense therapy because of our biases. She chose AC
docetaxel or dose-dense therapy — in that order, with de-emphasis on
the dose-dense therapy because of our biases. She chose AC![]() docetaxel.
docetaxel.
Tracks 22-24

![]() DR JAHANZEB: This is aggressive disease in a young patient, so I would like to
be aggressive and use a combination regimen. Because she has HER2-positive
disease, I would use trastuzumab in combination with chemotherapy. The next
question is, will it be with single-agent or combination chemotherapy?
DR JAHANZEB: This is aggressive disease in a young patient, so I would like to
be aggressive and use a combination regimen. Because she has HER2-positive
disease, I would use trastuzumab in combination with chemotherapy. The next
question is, will it be with single-agent or combination chemotherapy?
The only trial that demonstrated a benefit with triplet therapy was Nick Robert’s study of paclitaxel/carboplatin/trastuzumab versus paclitaxel/trastuzumab. Although the trial wasn’t powered and didn’t show a survival benefit, it did show a benefit in time to progression (Robert 2006), which can also be important. I would be tempted to talk to her about that triplet therapy.
![]() DR LOVE: MJ, what are your thoughts on docetaxel/carboplatin/trastuzumab
(TCH) for this patient?
DR LOVE: MJ, what are your thoughts on docetaxel/carboplatin/trastuzumab
(TCH) for this patient?
![]() DR JAHANZEB: I would have talked to her about TCH. If we wanted to use
just a single agent, which is my personal bias because of the results of our
Phase II trial of vinorelbine/trastuzumab (Jahanzeb 2002), we would use
vinorelbine to spare her the alopecia, the steroids and the neurotoxicity.
DR JAHANZEB: I would have talked to her about TCH. If we wanted to use
just a single agent, which is my personal bias because of the results of our
Phase II trial of vinorelbine/trastuzumab (Jahanzeb 2002), we would use
vinorelbine to spare her the alopecia, the steroids and the neurotoxicity.
![]() DR LOVE: What about the role of hormonal therapy?
DR LOVE: What about the role of hormonal therapy?
![]() DR JAHANZEB: Not concurrently. Once the disease is under control, for
maintenance, then the question would have been, would you continue trastuzumab?
Would you simply continue trastuzumab or add hormones at that time?
DR JAHANZEB: Not concurrently. Once the disease is under control, for
maintenance, then the question would have been, would you continue trastuzumab?
Would you simply continue trastuzumab or add hormones at that time?
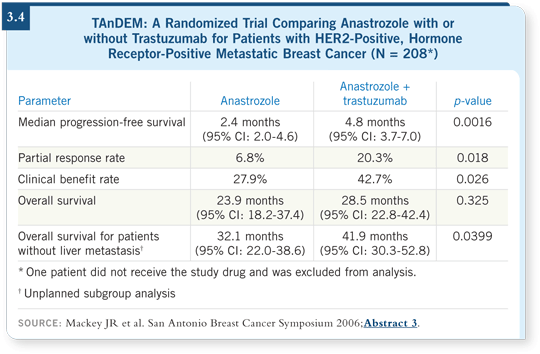
This patient is premenopausal, and we have no evidence supporting tamoxifen with trastuzumab. If she were postmenopausal after chemotherapy or we rendered her postmenopausal by ovarian ablation, we could consider the TAnDEM trial data showing that anastrozole with trastuzumab at least doubled the time to progression compared to anastrozole alone (Mackey 2006; [3.4]).
![]() DR LOVE: How did you end up treating this patient?
DR LOVE: How did you end up treating this patient?
![]() DR DICKLER: She was offered participation in our Phase II clinical trial of
nab paclitaxel, carboplatin and trastuzumab. She did extremely well with that regimen. She had a partial response and complete normalization of the breast.
The left axillary node shrank, and her pain was reduced.
DR DICKLER: She was offered participation in our Phase II clinical trial of
nab paclitaxel, carboplatin and trastuzumab. She did extremely well with that regimen. She had a partial response and complete normalization of the breast.
The left axillary node shrank, and her pain was reduced.
She did have an unusual reaction to carboplatin, with intense pain during the infusion in her lower extremities and some flushing. It occurred several infusions into her treatment. Carboplatin was stopped and she continued on nab paclitaxel and trastuzumab until a maximal response was detected, followed by ovarian suppression and letrozole.
EDITOR
Neil Love, MD
INTERVIEWS
Sharon Giordano, MD, MPH
- Select publications
Rowan T Chlebowski, MD, PhD
- Select publications
Tumor Panel Case Discussion
- Select publications
INTERVIEWS (continued)
Jack Cuzick, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity