
 |
||||||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss your 2006 ASCO presentation regarding the
rates of congestive heart failure among older women with breast cancer
(Giordano 2006a)?
DR LOVE: Can you discuss your 2006 ASCO presentation regarding the
rates of congestive heart failure among older women with breast cancer
(Giordano 2006a)?
![]() DR GIORDANO: We examined the SEER-Medicare database, which is a large
national database of people with cancer who are 65 years of age and older, to determine how people were treated in the adjuvant setting. Then we
compared the rates of heart failure at five and 10 years. Although the patients
who received anthracyclines tended to be younger and healthier, they had
higher rates of heart failure than patients who received other kinds of chemotherapy,
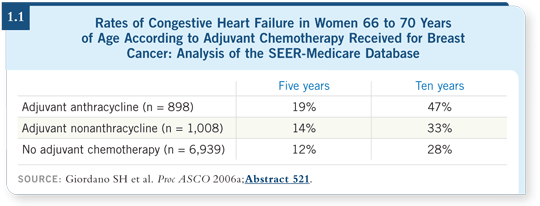
such as CMF (Giordano 2006a; [1.1]).
DR GIORDANO: We examined the SEER-Medicare database, which is a large
national database of people with cancer who are 65 years of age and older, to determine how people were treated in the adjuvant setting. Then we
compared the rates of heart failure at five and 10 years. Although the patients
who received anthracyclines tended to be younger and healthier, they had
higher rates of heart failure than patients who received other kinds of chemotherapy,
such as CMF (Giordano 2006a; [1.1]).
We saw high rates of congestive heart failure, even among patients who were not treated with any chemotherapy and those treated with nonanthracycline-based chemotherapy (Giordano 2006a; [1.1]). They are based on Medicare coding, which is an important caveat to remember. We don’t have ECHO results or physician examinations — we have billing codes from Medicare to indicate heart failure. However, studies have shown that this is a valid and accurate way to assess heart failure occurrence.
![]() DR LOVE: How much of an additional risk of congestive heart failure do you
think is introduced by an anthracycline?
DR LOVE: How much of an additional risk of congestive heart failure do you
think is introduced by an anthracycline?
![]() DR GIORDANO: An increase in risk of about five to 10 percent is associated
with receiving an adjuvant anthracycline, which is substantial.
DR GIORDANO: An increase in risk of about five to 10 percent is associated
with receiving an adjuvant anthracycline, which is substantial.
![]() DR LOVE: Most oncologists tell their patients that it is one percent.
DR LOVE: Most oncologists tell their patients that it is one percent.
![]() DR GIORDANO: That one percent figure is based on clinical trials evaluating
the short-term toxicities of the anthracyclines. Our numbers are based on five- and
10-year rates.
DR GIORDANO: That one percent figure is based on clinical trials evaluating
the short-term toxicities of the anthracyclines. Our numbers are based on five- and
10-year rates.
![]() DR LOVE: Are you telling your patients that by using an anthracycline-containing
regimen (ie, four cycles of AC), you’re creating an excess risk of
heart failure of five or 10 percent in the long run?
DR LOVE: Are you telling your patients that by using an anthracycline-containing
regimen (ie, four cycles of AC), you’re creating an excess risk of
heart failure of five or 10 percent in the long run?
![]() DR GIORDANO: Because these data are not from a randomized controlled
trial, I cannot say it with that degree of certainty. I am telling them that we
currently don’t have an accurate way of estimating the risk.
DR GIORDANO: Because these data are not from a randomized controlled
trial, I cannot say it with that degree of certainty. I am telling them that we
currently don’t have an accurate way of estimating the risk.
Track 5
![]() DR LOVE: What about trastuzumab and cardiac toxicity?
DR LOVE: What about trastuzumab and cardiac toxicity?
![]() DR GIORDANO: Clearly trastuzumab increases the risk of cardiac toxicity.
DR GIORDANO: Clearly trastuzumab increases the risk of cardiac toxicity.
Administering trastuzumab concurrently with chemotherapy seems to incur a higher risk than administering them sequentially, but you’re balancing that against trastuzumab perhaps being more effective when administered concurrently than sequentially to chemotherapy.
It’s frequently an issue that arises, especially for the patients with low-risk disease. For those patients — similar to using TC (docetaxel/cyclophosphamide) for patients with HER2-negative disease and a high risk of cardiac toxicity — I’ll often use TCH (docetaxel/carboplatin/trastuzumab) for the patients with HER2-positive disease and a high risk of cardiac toxicity.
![]() DR LOVE: What is your opinion of the data from BCIRG 006 with TCH
(Slamon 2006)?
DR LOVE: What is your opinion of the data from BCIRG 006 with TCH
(Slamon 2006)?
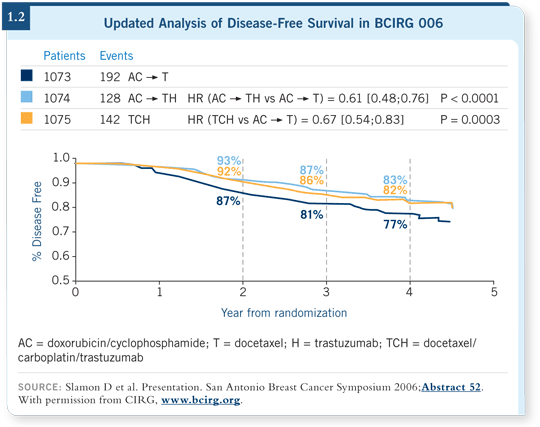
![]() DR GIORDANO: The evidence appears stronger and stronger that TCH is
equivalent to the anthracycline-based regimens (Slamon 2006; [1.2]). If I had
a patient who was older or one with pre-existing heart disease for whom I was
worried about cardiac toxicity, I would first use TCH.
DR GIORDANO: The evidence appears stronger and stronger that TCH is
equivalent to the anthracycline-based regimens (Slamon 2006; [1.2]). If I had
a patient who was older or one with pre-existing heart disease for whom I was
worried about cardiac toxicity, I would first use TCH.
![]() DR LOVE: What about patients with pre-existing hypertension or diabetes?
DR LOVE: What about patients with pre-existing hypertension or diabetes?
![]() DR GIORDANO: Those factors might weigh into my decision, but neither one
of them by itself would sway me one way or the other. Patients with a borderline
ejection fraction of 50 or 55 percent who clinically appear to be well and
perhaps don’t have any cardiac risk factors and with whom you want to use
chemotherapy also are great candidates for TCH.
DR GIORDANO: Those factors might weigh into my decision, but neither one
of them by itself would sway me one way or the other. Patients with a borderline
ejection fraction of 50 or 55 percent who clinically appear to be well and
perhaps don’t have any cardiac risk factors and with whom you want to use
chemotherapy also are great candidates for TCH.
Track 4
![]() DR LOVE: What’s your opinion about the use of adjuvant chemotherapy
for patients with ER-positive tumors?
DR LOVE: What’s your opinion about the use of adjuvant chemotherapy
for patients with ER-positive tumors?
![]() DR GIORDANO: Increasingly, data suggest that patients with ER-positive
tumors benefit less from chemotherapy than patients with ER-negative
tumors, but I’m not convinced at this point that they receive no benefit.
Studies like TAILORx (1.3), in which we are stratifying patients with ER-positive
disease by risk as determined with the Oncotype DX assay, might help
establish whether there is a subset of patients who don’t need chemotherapy.
For patients who are borderline candidates for chemotherapy, if they have ER-positive
disease, I’m less inclined to use chemotherapy. I believe, however, that
patients who have high-risk disease, even if it’s ER-positive, still clearly benefit.
DR GIORDANO: Increasingly, data suggest that patients with ER-positive
tumors benefit less from chemotherapy than patients with ER-negative
tumors, but I’m not convinced at this point that they receive no benefit.
Studies like TAILORx (1.3), in which we are stratifying patients with ER-positive
disease by risk as determined with the Oncotype DX assay, might help
establish whether there is a subset of patients who don’t need chemotherapy.
For patients who are borderline candidates for chemotherapy, if they have ER-positive
disease, I’m less inclined to use chemotherapy. I believe, however, that
patients who have high-risk disease, even if it’s ER-positive, still clearly benefit.
![]() DR LOVE: Are you enrolling patients on TAILORx, and how do you use the
Oncotype DX assay off protocol?
DR LOVE: Are you enrolling patients on TAILORx, and how do you use the
Oncotype DX assay off protocol?
![]() DR GIORDANO: We do participate in TAILORx, which runs the Oncotype
DX assay for the patient. If a patient’s tumor is categorized as presenting
intermediate risk, then she is randomly assigned to hormonal therapy with
or without chemotherapy. If the patient’s tumor is in the low-risk category,
she receives hormonal therapy alone. If the patient’s tumor is in the high-risk
category, she receives chemotherapy and hormonal therapy.
DR GIORDANO: We do participate in TAILORx, which runs the Oncotype
DX assay for the patient. If a patient’s tumor is categorized as presenting
intermediate risk, then she is randomly assigned to hormonal therapy with
or without chemotherapy. If the patient’s tumor is in the low-risk category,
she receives hormonal therapy alone. If the patient’s tumor is in the high-risk
category, she receives chemotherapy and hormonal therapy.
I’ve used the Oncotype DX assay off protocol — to help both myself and the patient make a decision as to whether to use chemotherapy — for patients who are borderline candidates for chemotherapy because they do not want chemotherapy or they have comorbidities or fairly low-risk tumors.
I can’t say that I have a clear cutoff, but for patients with tumors larger than two centimeters, I’m inclined to use chemotherapy. I typically don’t order the Oncotype DX assay in that situation.
Tracks 8, 10
![]() DR LOVE: What do we know about adjuvant endocrine therapy for men
with breast cancer?
DR LOVE: What do we know about adjuvant endocrine therapy for men
with breast cancer?
![]() DR GIORDANO: Not enough. More than 90 percent of men with breast cancer
have hormone receptor-positive disease (Giordano 2004). When I’m selecting
adjuvant hormonal therapies, I almost exclusively use tamoxifen because we
have strong data in the metastatic setting with male patients, and we have
retrospective series showing a survival benefit for men treated with adjuvant
tamoxifen (Goss 1999; Giordano 2005; Fentiman 2006). None of the data are
at the level of a randomized trial, but at least we have solid data. The controversy
comes in with the use of the aromatase inhibitors and whether they
are going to be equally, less or more effective than tamoxifen. You can make
arguments in any direction about how effective they might be. Most of the estrogen in men comes from peripheral aromatization, so it makes sense that if
you could shut that down with aromatase inhibitors, it may be effective.
DR GIORDANO: Not enough. More than 90 percent of men with breast cancer
have hormone receptor-positive disease (Giordano 2004). When I’m selecting
adjuvant hormonal therapies, I almost exclusively use tamoxifen because we
have strong data in the metastatic setting with male patients, and we have
retrospective series showing a survival benefit for men treated with adjuvant
tamoxifen (Goss 1999; Giordano 2005; Fentiman 2006). None of the data are
at the level of a randomized trial, but at least we have solid data. The controversy
comes in with the use of the aromatase inhibitors and whether they
are going to be equally, less or more effective than tamoxifen. You can make
arguments in any direction about how effective they might be. Most of the estrogen in men comes from peripheral aromatization, so it makes sense that if
you could shut that down with aromatase inhibitors, it may be effective.

However, when anastrozole was first being developed, it was tested in healthy male volunteers. Those patients exhibited a decline in estrogen levels — although not as complete as the decline among women — but because of the feedback loop, they also experienced a doubling of their testosterone levels (Mauras 2000).
The bottom line is that we have a couple of case reports with responses to the aromatase inhibitors (Zabolotny 2005; Italiano 2004). We have a case series of five patients in which a few had stable disease but no responses were recorded (Giordano 2002). So few data are available on their efficacy that I’m reluctant to use them in the adjuvant setting.
![]() DR LOVE: What about the LHRH agonists?
DR LOVE: What about the LHRH agonists?
![]() DR GIORDANO: Those agents are likely to be effective. I’ve had patients
who responded nicely to LHRH agonists in the metastatic setting, and I’ve
had some good responses with the combination of an LHRH agonist and
an aromatase inhibitor. This makes sense because you’re shutting back the
negative feedback loop in addition to shutting off the aromatase.
DR GIORDANO: Those agents are likely to be effective. I’ve had patients
who responded nicely to LHRH agonists in the metastatic setting, and I’ve
had some good responses with the combination of an LHRH agonist and
an aromatase inhibitor. This makes sense because you’re shutting back the
negative feedback loop in addition to shutting off the aromatase.
We published a letter to the editor in the Journal of Clinical Oncology in which we reported two cases. One of them was interesting because the patient had failed leuprolide and an aromatase inhibitor as single agents but responded to the combination (Giordano 2006b).
![]() DR LOVE: What about adjuvant chemotherapy in male breast cancer? Do we
have any data?
DR LOVE: What about adjuvant chemotherapy in male breast cancer? Do we
have any data?
![]() DR GIORDANO: We have minimal data. One prospective study from the NCI
treated about 30 men who had Stage II, node-positive breast cancer with CMF
and then compared the outcomes to historical controls. This study indicated a
better survival than expected compared to the historical controls (Bagley 1987;
Walshe 2007).
DR GIORDANO: We have minimal data. One prospective study from the NCI
treated about 30 men who had Stage II, node-positive breast cancer with CMF
and then compared the outcomes to historical controls. This study indicated a
better survival than expected compared to the historical controls (Bagley 1987;
Walshe 2007).
We’ve retrospectively analyzed the MD Anderson experience (Giordano 2005). It appears that survival is a little better, but we have no data to guide us. I believe it’s reasonable to expect that chemotherapy would be the same for male and female patients, and I use the same regimens.
Track 14
![]() DR LOVE: I’m curious about your opinion of the data on bevacizumab
with paclitaxel that came out a couple of years ago from ECOG-E2100
(Miller 2005). How are you approaching the management of metastatic
disease, specifically the use of chemotherapy and bevacizumab?
DR LOVE: I’m curious about your opinion of the data on bevacizumab
with paclitaxel that came out a couple of years ago from ECOG-E2100
(Miller 2005). How are you approaching the management of metastatic
disease, specifically the use of chemotherapy and bevacizumab?
![]() DR GIORDANO: I’m discussing bevacizumab in combination with taxanes
with patients up front in the first-line metastatic setting. I haven’t been using
it in the second-, third- or fourth-line setting. I know ECOG-E2100 included
patients who had received adjuvant taxanes, but often taxanes aren’t my first
choice in the up-front metastatic setting because so many of my patients have
already received them as adjuvant therapy. I usually start with capecitabine.
DR GIORDANO: I’m discussing bevacizumab in combination with taxanes
with patients up front in the first-line metastatic setting. I haven’t been using
it in the second-, third- or fourth-line setting. I know ECOG-E2100 included
patients who had received adjuvant taxanes, but often taxanes aren’t my first
choice in the up-front metastatic setting because so many of my patients have
already received them as adjuvant therapy. I usually start with capecitabine.
Track 17
![]() DR LOVE: How do you approach the initiation of adjuvant endocrine
therapy for the postmenopausal patient?
DR LOVE: How do you approach the initiation of adjuvant endocrine
therapy for the postmenopausal patient?
![]() DR GIORDANO: I start up front with an aromatase inhibitor. My belief is that
the data show it’s a more effective medication. I recognize that we haven’t
directly compared starting with tamoxifen and switching to an aromatase
inhibitor to starting with an aromatase inhibitor. However, the data we have
show that the aromatase inhibitors are better, and I have a concern about the patients who would relapse in the period when they’re receiving tamoxifen
who might not relapse if I had started with an aromatase inhibitor. So my
preference is to start with an aromatase inhibitor.
DR GIORDANO: I start up front with an aromatase inhibitor. My belief is that
the data show it’s a more effective medication. I recognize that we haven’t
directly compared starting with tamoxifen and switching to an aromatase
inhibitor to starting with an aromatase inhibitor. However, the data we have
show that the aromatase inhibitors are better, and I have a concern about the patients who would relapse in the period when they’re receiving tamoxifen
who might not relapse if I had started with an aromatase inhibitor. So my
preference is to start with an aromatase inhibitor.
![]() DR LOVE: How do you approach the postmenopausal patient who has received
five years of tamoxifen, particularly those women who may have been off
therapy for one, two, three or four years?
DR LOVE: How do you approach the postmenopausal patient who has received
five years of tamoxifen, particularly those women who may have been off
therapy for one, two, three or four years?
![]() DR GIORDANO: If they’ve been off tamoxifen for six months or a year, then
I would probably start an aromatase inhibitor as extended adjuvant therapy. I
don’t feel we have enough data for starting an aromatase inhibitor two, three
or four years after. This is a sort of “do no harm” principle because toxicity
is associated with the aromatase inhibitors, especially bone loss, and we don’t
have high-quality evidence available for that group of patients.
DR GIORDANO: If they’ve been off tamoxifen for six months or a year, then
I would probably start an aromatase inhibitor as extended adjuvant therapy. I
don’t feel we have enough data for starting an aromatase inhibitor two, three
or four years after. This is a sort of “do no harm” principle because toxicity
is associated with the aromatase inhibitors, especially bone loss, and we don’t
have high-quality evidence available for that group of patients.
Having said that, I wonder if a benefit might exist. When we’ve previously observed recurrence rates for ER-positive breast cancer, the hazard each year flattens out indefinitely. At 10 years, a person still does have a significant risk.
EDITOR
Neil Love, MD
INTERVIEWS
Sharon Giordano, MD, MPH
- Select publications
Rowan T Chlebowski, MD, PhD
- Select publications
Tumor Panel Case Discussion
- Select publications
INTERVIEWS (continued)
Jack Cuzick, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity