
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3
![]() DR LOVE: Could you review the meta-analysis you published regarding
adjuvant LHRH agonists in premenopausal patients with hormone
receptor-positive breast cancer (LHRH-Agonists Overview Group 2007)?
DR LOVE: Could you review the meta-analysis you published regarding
adjuvant LHRH agonists in premenopausal patients with hormone
receptor-positive breast cancer (LHRH-Agonists Overview Group 2007)?
![]() DR CUZICK: The LHRH agonists are a much-underused treatment. The trials
have been around for some time but, individually, none of the trials has been
convincing regarding what constitutes their best use. We managed to get all
the individual patient data from virtually every trial in the world, and we addressed two major questions: Are the LHRH agonists, when used alone, as
effective as chemotherapy and second, if used in addition to chemotherapy, do
they provide any additional benefit?
DR CUZICK: The LHRH agonists are a much-underused treatment. The trials
have been around for some time but, individually, none of the trials has been
convincing regarding what constitutes their best use. We managed to get all
the individual patient data from virtually every trial in the world, and we addressed two major questions: Are the LHRH agonists, when used alone, as
effective as chemotherapy and second, if used in addition to chemotherapy, do
they provide any additional benefit?
![]() DR LOVE: The other question that oncologists have is how LHRH agonists
compare to tamoxifen alone.
DR LOVE: The other question that oncologists have is how LHRH agonists
compare to tamoxifen alone.
![]() DR CUZICK: No direct comparisons to tamoxifen have been conducted, but
some evidence emerged that LHRH agonists with tamoxifen added benefit
compared to tamoxifen alone.
DR CUZICK: No direct comparisons to tamoxifen have been conducted, but
some evidence emerged that LHRH agonists with tamoxifen added benefit
compared to tamoxifen alone.
Unfortunately, no trials have addressed the most relevant question: If everybody receives tamoxifen, is adding an LHRH agonist the same as adding chemotherapy? This is unfortunate because it is a key question right now.
This overview was focused on chemical castration with LHRH agonists (4.1), and the trials were grouped into three classes. A few patients received an LHRH agonist versus nothing — only 338 patients. The effects were as predicted but not quite significant because of the small numbers. They demonstrated approximately a 30 percent reduction in recurrence and mortality.
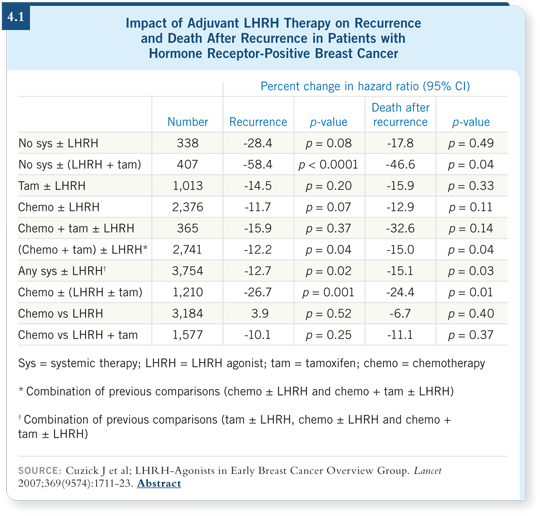
The next group of trials directly evaluated an LHRH agonist versus chemotherapy. Most of the chemotherapy was CMF, but some anthracycline-based regimens were administered. Almost 4,000 patients were in that grouping, and the evidence suggested that they were essentially equally effective.
One of the limitations is that the chemotherapy was from an older era, but in fact, we could find no real difference between the anthracycline-based and the CMF-based chemotherapy. It does suggest that, certainly for women with estrogen receptor-positive, low-risk cancer, an LHRH agonist with tamoxifen is a reasonable option and chemotherapy isn’t necessary for all those patients.
The third round of trials evaluated the addition of an LHRH agonist to chemotherapy, tamoxifen or both. The effects were modest — a 10 to 15 percent improvement in recurrence and mortality — and that was true whether it was added to tamoxifen, chemotherapy or both.
![]() DR LOVE: In the studies of LHRH agonists in breast cancer, was the interval
used for ovarian suppression one month or three months?
DR LOVE: In the studies of LHRH agonists in breast cancer, was the interval
used for ovarian suppression one month or three months?
![]() DR CUZICK: These were almost exclusively with monthly injections. A lot of
interest is shown in that question because it would be much more convenient if
it were three monthly.
DR CUZICK: These were almost exclusively with monthly injections. A lot of
interest is shown in that question because it would be much more convenient if
it were three monthly.
The data I’ve seen suggest that many, but not all, women can achieve ovarian suppression with three-monthly injections. The results are somewhat variable, and enough uncertainty exists that three-monthly injections are not typically recommended.
Track 6
![]() DR LOVE: Controversy has existed for the last five or six years about the
optimal sequence or the optimal endocrine therapy for postmenopausal
women. Can you discuss the model you developed and where you believe
this is heading (Cuzick 2007a; [4.2])?
DR LOVE: Controversy has existed for the last five or six years about the
optimal sequence or the optimal endocrine therapy for postmenopausal
women. Can you discuss the model you developed and where you believe
this is heading (Cuzick 2007a; [4.2])?
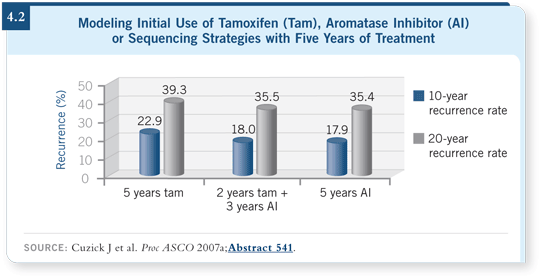
![]() DR CUZICK: We’ve produced a model that evaluates and updates the data
based on the most recent trial results. The model predicts that up-front treatment
with an aromatase inhibitor will be the best strategy, in terms of both
efficacy and toxicity.
DR CUZICK: We’ve produced a model that evaluates and updates the data
based on the most recent trial results. The model predicts that up-front treatment
with an aromatase inhibitor will be the best strategy, in terms of both
efficacy and toxicity.
The efficacy benefits of the up-front aromatase inhibitor over the switching strategy are modest, but I believe a lot of confusion has occurred, specifically about comparing the up-front treatments directly to the switching strategies, because the switching trials only evaluate patients that completed two years of treatment without side effects or a recurrence. It’s a different population from the population receiving the up-front strategy.
Track 8
![]() DR LOVE: In our Patterns of Care studies, we’ve seen a progressive shift
since the 2001 publication of the ATAC data toward the use of up-front
aromatase inhibitors.
DR LOVE: In our Patterns of Care studies, we’ve seen a progressive shift
since the 2001 publication of the ATAC data toward the use of up-front
aromatase inhibitors.
That clearly is now the dominant initial strategy, at least in the United
States, but a pocket of investigators still believe that tamoxifen should be
considered up front for a couple of years for patients with node-negative
disease. Do you agree or disagree with that?
![]() DR CUZICK: I disagree. The only value of tamoxifen is for patients who do not
tolerate the aromatase inhibitors. A subgroup of patients develop problematic
arthralgias, but by and large the side effects of tamoxifen are worse than the
side effects of the aromatase inhibitors.
DR CUZICK: I disagree. The only value of tamoxifen is for patients who do not
tolerate the aromatase inhibitors. A subgroup of patients develop problematic
arthralgias, but by and large the side effects of tamoxifen are worse than the
side effects of the aromatase inhibitors.
The number of patients stopping treatment because of side effects was substantially and significantly higher with tamoxifen than with the aromatase inhibitors. We know problems are associated with tamoxifen, such as thromboembolic events and a range of gynecological problems, including endometrial cancer and a four times higher rate of hysterectomies (Duffy 2005, 2006).
So for patients with low-risk disease, the issues are more related to safety and tolerability than efficacy, and overall, the aromatase inhibitors are better. Clearly toxicities do occur, to the extent that some patients cannot tolerate these agents, and in those cases, tamoxifen is better than nothing.
For patients with higher-risk disease, the efficacy profile is most important. I can’t, therefore, identify a group of patients for whom I wouldn’t want to start with an aromatase inhibitor.
Track 15
![]() DR LOVE: Can you review the IBIS-1 tamoxifen prevention trial data?
DR LOVE: Can you review the IBIS-1 tamoxifen prevention trial data?
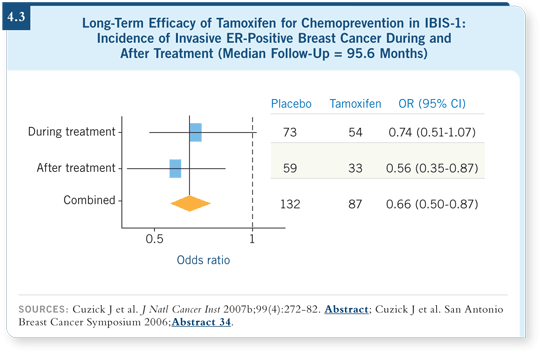
![]() DR CUZICK: The IBIS-1 study began in 1994 and demonstrated essentially a
50 percent reduction in new ER-positive breast cancer, with no effect on ER-negative
tumors (Cuzick 2002).
DR CUZICK: The IBIS-1 study began in 1994 and demonstrated essentially a
50 percent reduction in new ER-positive breast cancer, with no effect on ER-negative
tumors (Cuzick 2002).
The European trials have kept themselves blinded. That study and the Royal Marsden study, which originally was the pilot study for IBIS-1, have been ongoing, and we recently updated the data with a median follow-up of eight years (Cuzick 2007b).
The results were exciting and as good as we could have ever hoped for. The benefit in the five years after stopping treatment was greater than the benefit during the five years of active treatment (4.3).
Track 17
![]() DR LOVE: Can you provide an update on data related to quality of life in
the ATAC study?
DR LOVE: Can you provide an update on data related to quality of life in
the ATAC study?
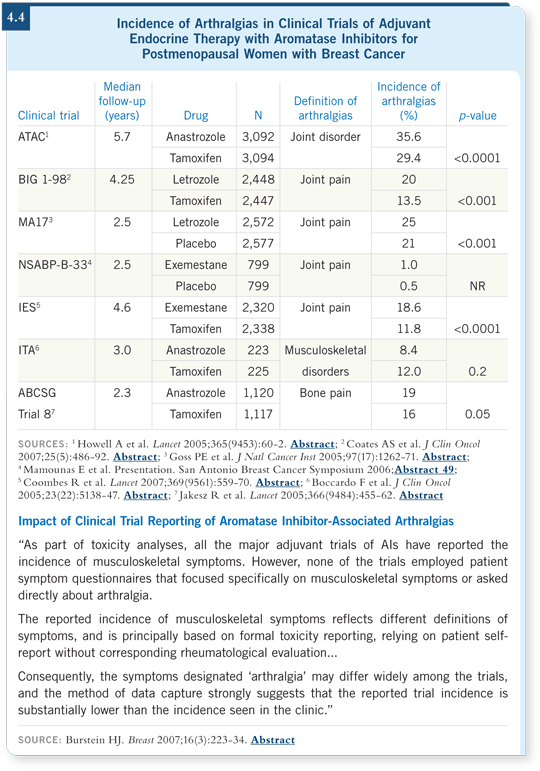
![]() DR CUZICK: Not surprisingly, we see somewhat but not enormously higher
rates of arthralgias with anastrozole.
DR CUZICK: Not surprisingly, we see somewhat but not enormously higher
rates of arthralgias with anastrozole.
The rate is 30 percent with tamoxifen and 36 percent with anastrozole (Buzdar 2006; [4.4]). So the effect is real, but it’s a small effect compared to the fact that arthralgia is not uncommon in the early postmenopausal years anyway.
So to some extent, the aromatase inhibitors are being blamed for some arthralgias that they don’t cause. They do increase the risk, but a lot of arthralgias will occur anyway.
We will learn more about that from the IBIS-2 study because we’ll be comparing anastrozole to placebo, and there’s no doubt that a fair amount of arthralgia is occurring in the placebo arm.
We have a nice paper that will be ready for San Antonio, in which we’ve analyzed the ATAC trial for the risk factors for arthralgia and its severity. There are risk factors for arthralgia that are stronger than treatment with an aromatase inhibitor.
Track 10
![]() DR LOVE: What are your thoughts about the duration of use of adjuvant
aromatase inhibitors?
DR LOVE: What are your thoughts about the duration of use of adjuvant
aromatase inhibitors?
![]() DR CUZICK: Duration of endocrine treatment is probably one of the key
questions now, particularly whether we should treat beyond five years.
DR CUZICK: Duration of endocrine treatment is probably one of the key
questions now, particularly whether we should treat beyond five years.
For the patient at average to higher risk, the question of five versus 10 years of treatment with an aromatase inhibitor is an important one.
![]() DR LOVE: What do you think is going on when you see drops in recurrence
rates when starting an aromatase inhibitor after five years of tamoxifen, maybe
even with a delay of a few years after completing tamoxifen?
DR LOVE: What do you think is going on when you see drops in recurrence
rates when starting an aromatase inhibitor after five years of tamoxifen, maybe
even with a delay of a few years after completing tamoxifen?
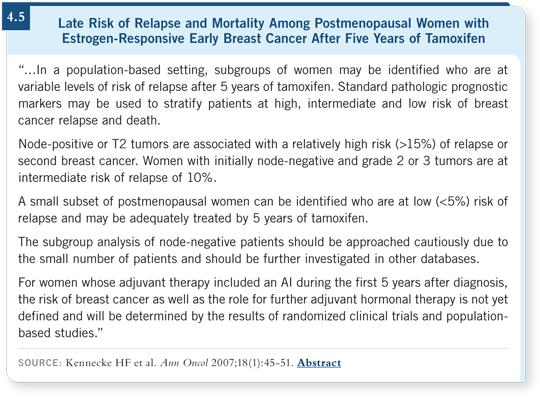
![]() DR CUZICK: Breast cancer is striking in that the recurrence rate never drops
below two percent per year, even out to 20 years. It’s a disease with a long
recurrence rate (4.5).
DR CUZICK: Breast cancer is striking in that the recurrence rate never drops
below two percent per year, even out to 20 years. It’s a disease with a long
recurrence rate (4.5).
It’s difficult to believe that those are all true recurrences. Many of those, to some extent, must be development of lesions that are not fully invasive at the time of diagnosis. So there’s a mixture of prevention and treatment.
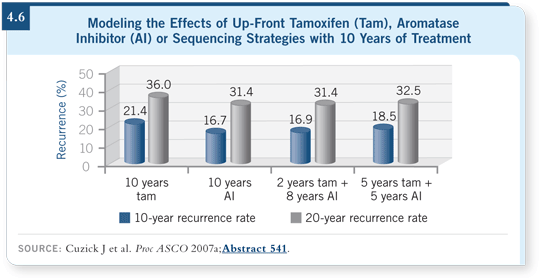
However, the data are impressive in indicating that longer treatment for many patients is likely to be beneficial. We’ve evaluated that in our modeling poster this year at ASCO and have also come to the conclusion that there are benefits to 10 years of treatment (Cuzick 2007a; [4.6]).
EDITOR
Neil Love, MD
INTERVIEWS
Sharon Giordano, MD, MPH
- Select publications
Rowan T Chlebowski, MD, PhD
- Select publications
Tumor Panel Case Discussion
- Select publications
INTERVIEWS (continued)
Jack Cuzick, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity