
 |
||||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Would you review the recent data on the decrease in breast
cancer incidence in the United States?
DR LOVE: Would you review the recent data on the decrease in breast
cancer incidence in the United States?
![]() DR CHLEBOWSKI: Over the last 20 years, the age-adjusted incidence of breast
cancer had been increasing at about half a percent per year until the last few
years.
DR CHLEBOWSKI: Over the last 20 years, the age-adjusted incidence of breast
cancer had been increasing at about half a percent per year until the last few
years.
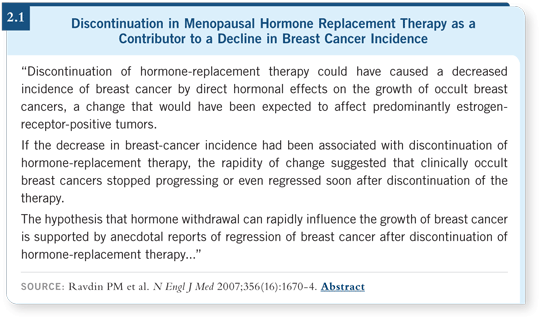
Between 2002 and 2003, data from the NCI’s SEER registries show approximately a seven percent reduction in the incidence (Ravdin 2007). Peter Ravdin, some other NCI investigators and I began examining the data to determine whether this was real and why it occurred.
We reviewed all of the SEER registries, which capture 26 percent of the cancer data in the United States, and all nine reported the same reduction. We examined the data for a month-to-month variation and found no fluctuation over the last 10 years.
In addition, we reviewed the data for 15 or 20 common types of cancer and found no change in any of them during this time period. When we examined subgroups, we found that almost all of the reduction occurred in patients with breast cancer in the ER-positive subgroup and among ages 50 to 69.
We examined the second-primary breast cancer cases to see if the statistics might be influenced by the use of tamoxifen and/or aromatase inhibitors, but those weren’t decreased. Indeed, it appears the reduction is real and it’s limited to postmenopausal women with ER-positive breast cancer.
![]() DR LOVE: To what do you attribute the reduction?
DR LOVE: To what do you attribute the reduction?
![]() DR CHLEBOWSKI: A few changes during this time frame could have accounted
for this reduction, and one was the use of mammography. In early 2000 to
2001, the Cochrane Collaboration challenged the effectiveness of mammography,
and major organizations examined whether this was truly an issue.
When the smoke cleared, they continued to support mammograms, but a
decrease of one percent occurred in mammography use across the country
between 2000 and 2003. The biggest decrease was among women ages 50 to
69 — a 3.2 percent decrease.
DR CHLEBOWSKI: A few changes during this time frame could have accounted
for this reduction, and one was the use of mammography. In early 2000 to
2001, the Cochrane Collaboration challenged the effectiveness of mammography,
and major organizations examined whether this was truly an issue.
When the smoke cleared, they continued to support mammograms, but a
decrease of one percent occurred in mammography use across the country
between 2000 and 2003. The biggest decrease was among women ages 50 to
69 — a 3.2 percent decrease.
Another change that occurred during this time was a decline in the use of hormone replacement therapy (HRT; [2.1]). In early 2002, reports on the use of estrogen with progesterone suggested that there was not a chronic disease benefit. Rather, there was a chronic disease risk associated with HRT, and a tremendous reaction occurred in the population. In the WHI estrogen with progesterone trial, we started with 16,000 women, but in the end we had 9,000 women still randomly assigned to placebo or estrogen with progesterone. Overnight, we informed the women in a letter to stop their study pills.
The number of HRT prescriptions in the United States decreased from 60 million in 2000 and 2002 to 25 million in 2003.
Track 9
![]() DR LOVE: Can you review the updated data from the WINS trial?
DR LOVE: Can you review the updated data from the WINS trial?
![]() DR CHLEBOWSKI: This randomized, prospective clinical trial involved mainly
postmenopausal women, ages 48 to 78 at entry, with early-stage, resected
breast cancer. It was conducted at 39 institutions throughout the United States,
and patients were randomly assigned to a dietary intervention targeting reduction
of fat intake or not.
DR CHLEBOWSKI: This randomized, prospective clinical trial involved mainly
postmenopausal women, ages 48 to 78 at entry, with early-stage, resected
breast cancer. It was conducted at 39 institutions throughout the United States,
and patients were randomly assigned to a dietary intervention targeting reduction
of fat intake or not.
All the women received standard breast cancer management. If the patient had receptor-negative disease, adjuvant chemotherapy was required. For patients with receptor-positive disease, it was elective.
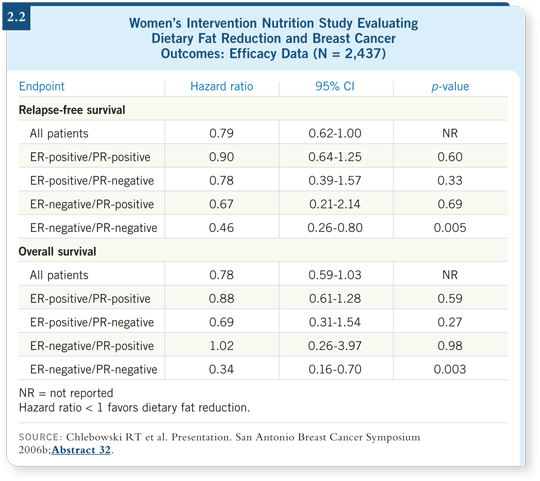
At ASCO 2005 I reported an interim efficacy analysis, which suggested an approximate 24 percent reduction in relapse-free survival, the primary study endpoint (Chlebowski 2006a). The hazard ratio for survival was 0.89, which was not significant.
At the San Antonio meeting in 2006, we presented a follow-up interim analysis (2.2). At that point, the relapse-free survival hazard ratio was 0.79, which was borderline, and the overall survival hazard ratio was 0.78, a 22 percent reduction in mortality, although not significant (Chlebowski 2006b).
In subgroup analysis, we saw statistically significant reductions in ER-negative, PR-negative cancer. In examining 362 such cases, we saw a 54 percent reduction in recurrence and a 66 percent reduction in mortality. This was an unplanned subgroup, but it is an interesting signal. When we compare the WHI to the WINS data, we see a similar trend with a doubling or tripling of the effect, especially among patients with PR-negative disease.
We don’t know whether any effect occurred in ER-positive tumors, because the hazard ratio for relapse-free survival in ER-positive, PR-positive breast cancer was 0.90. Although it’s in the right direction, we need further follow-up.
Track 13
![]() DR LOVE: What are your thoughts on extended adjuvant endocrine
therapy?
DR LOVE: What are your thoughts on extended adjuvant endocrine
therapy?
![]() DR CHLEBOWSKI: It’s true that hormone receptor-negative breast cancer
recurs more frequently early and then less commonly after three or four years
than hormone receptor-positive breast cancer, and then the recurrence tails
continue for a long period. The concern about the hormone receptor-positive
tail is legitimate, so I believe we are headed toward longer-duration hormonal
therapy.
DR CHLEBOWSKI: It’s true that hormone receptor-negative breast cancer
recurs more frequently early and then less commonly after three or four years
than hormone receptor-positive breast cancer, and then the recurrence tails
continue for a long period. The concern about the hormone receptor-positive
tail is legitimate, so I believe we are headed toward longer-duration hormonal
therapy.
![]() DR LOVE: How do you estimate a patient’s residual risk of relapse when
considering further therapy?
DR LOVE: How do you estimate a patient’s residual risk of relapse when
considering further therapy?
![]() DR CHLEBOWSKI: It’s difficult to calculate the patient’s risk of recurrence
because there are so many variables.
DR CHLEBOWSKI: It’s difficult to calculate the patient’s risk of recurrence
because there are so many variables.
Data from a variety of trials tell us that about half of the recurrence risk and only a third of the risk of dying is in the first five years.
It comes back to the questions of what our threshold is for treatment side effects to warrant an intervention and what is the toxicity of that intervention. In terms of aromatase inhibitors, and perhaps especially anastrozole, the toxicity profile seems to be settling so well that I am less concerned about administering a longer-duration therapy.
Track 14
![]() DR LOVE: Can you review the available data on up-front aromatase
inhibitors?
DR LOVE: Can you review the available data on up-front aromatase
inhibitors?
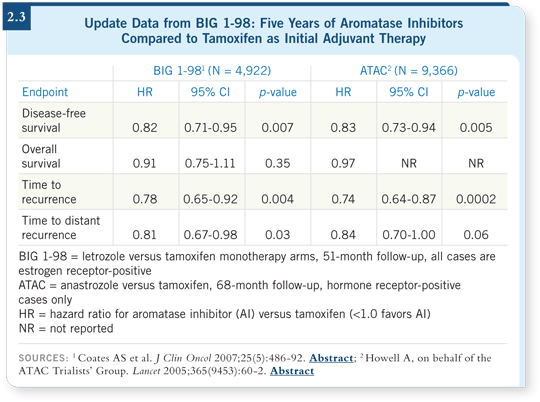
![]() DR CHLEBOWSKI: The data we have in the front-line setting with anastrozole
in the ATAC trial and letrozole in the BIG 1-98 trial are the easiest to
compare (2.3).
DR CHLEBOWSKI: The data we have in the front-line setting with anastrozole
in the ATAC trial and letrozole in the BIG 1-98 trial are the easiest to
compare (2.3).
The BIG 1-98 trial has four arms, but the 51-month update compares the letrozole to the tamoxifen monotherapy arms only, not the crossover arms. In this update, the hazard ratio for time to distant recurrence, which was 0.73 in the 26-month report, is now 0.81 (BIG 1-98 Collaborative Group 2005; Coates 2007). In the ATAC trial at 68 months, we see a hazard ratio of 0.84 (Howell 2005).
Track 15
![]() DR LOVE: What did the BIG 1-98 data show with regard to letrozole and
cardiovascular disease, and how does that compare to the other aromatase
inhibitors?
DR LOVE: What did the BIG 1-98 data show with regard to letrozole and
cardiovascular disease, and how does that compare to the other aromatase
inhibitors?
![]() DR CHLEBOWSKI: The BIG 1-98 trial had a higher rate of Grade III, IV and
V events and deaths with letrozole versus tamoxifen. However, it’s difficult to
make cross-study comparisons of some of these endpoints. In BIG 1-98, they
asked more specifically about some of the cardiac toxicities.
DR CHLEBOWSKI: The BIG 1-98 trial had a higher rate of Grade III, IV and
V events and deaths with letrozole versus tamoxifen. However, it’s difficult to
make cross-study comparisons of some of these endpoints. In BIG 1-98, they
asked more specifically about some of the cardiac toxicities.
I believe letrozole brings the potential for a slight increase of coronary artery disease, but whether that’s a substantial difference or related to the peculiarities of the BIG 1-98 trial is difficult to know.
A reasonable person could say that the MA17 data are more reliable estimates because it’s placebo controlled, and I don’t believe any effect has been seen.
![]() DR LOVE: What about the incidence of strokes?
DR LOVE: What about the incidence of strokes?
![]() DR CHLEBOWSKI: Anastrozole is the only one of the three aromatase inhibitors
that has shown a reduction in the rate of strokes in the clinical trials when
compared to tamoxifen.
DR CHLEBOWSKI: Anastrozole is the only one of the three aromatase inhibitors
that has shown a reduction in the rate of strokes in the clinical trials when
compared to tamoxifen.
In the ATAC trial, the incidence of ischemic cerebrovascular events was two percent among patients on anastrozole and 2.8 percent among patients on tamoxifen, which was statistically significant (Howell 2005).
Tracks 16-18
![]() DR LOVE: What about aromatase inhibitors and bone density?
DR LOVE: What about aromatase inhibitors and bone density?
![]() DR CHLEBOWSKI: It’s difficult for me to be particularly concerned about the
risk of fractures with aromatase inhibitors.
DR CHLEBOWSKI: It’s difficult for me to be particularly concerned about the
risk of fractures with aromatase inhibitors.
Per Lonning conducted a trial in early-stage breast cancer, evaluating exemestane versus placebo for two years. At the one-year follow-up, the bone had returned to normal in the spine and was returning to normal in the hip, with no intervention (Lonning 2005; Geisler 2006). That gave us the first signal that this complication is self correcting, even if you do nothing.
The second signal was in the ATAC trial, which involved no pretreatment, no screening and no calcium/vitamin D or protocol-defined bisphosphonate therapy.
The 68-month data showed the hip fracture rate was similar among the patients who received anastrozole versus tamoxifen — 1.2 versus one percent, respectively (Coleman 2006).
![]() DR LOVE: What about the bisphosphonates and recurrence?
DR LOVE: What about the bisphosphonates and recurrence?
![]() DR CHLEBOWSKI: In the update from Z-FAST/ZO-FAST, which compares
the use of bisphosphonates for patients on aromatase inhibitors up front to
delayed therapy, we saw a signal that fewer breast cancer recurrences might
occur among patients who receive this therapy, even in the delayed setting
(Brufsky 2006a).
DR CHLEBOWSKI: In the update from Z-FAST/ZO-FAST, which compares
the use of bisphosphonates for patients on aromatase inhibitors up front to
delayed therapy, we saw a signal that fewer breast cancer recurrences might
occur among patients who receive this therapy, even in the delayed setting
(Brufsky 2006a).
![]() DR LOVE: What was your take on bone density data from the ATAC trial
presented at ASCO in 2006 (Coleman 2006)?
DR LOVE: What was your take on bone density data from the ATAC trial
presented at ASCO in 2006 (Coleman 2006)?
![]() DR CHLEBOWSKI: The ATAC data show approximately an eight percent
difference in bone mineral density, which is statistically significant, but one
has to remember just how much bone loss you need to drop a T-score — it’s
10 to 12 percent. To go from normal to osteoporosis, it’s 20 to 25 percent
bone loss. They aren’t big numbers.
DR CHLEBOWSKI: The ATAC data show approximately an eight percent
difference in bone mineral density, which is statistically significant, but one
has to remember just how much bone loss you need to drop a T-score — it’s
10 to 12 percent. To go from normal to osteoporosis, it’s 20 to 25 percent
bone loss. They aren’t big numbers.
However, none of the patients in the ATAC trial who had normal bone mineral density developed osteoporosis with five years of aromatase inhibitor therapy. When I examine the accumulated data, it seems unlikely that we would cause any hip fractures treating 50- and 60-year-olds with aromatase inhibitors for five years.
![]() DR LOVE: What’s your approach to monitoring bone density and using
bisphosphonates for patients on aromatase inhibitors?
DR LOVE: What’s your approach to monitoring bone density and using
bisphosphonates for patients on aromatase inhibitors?
![]() DR CHLEBOWSKI: I’m involved in the process of updating the ASCO bone
health guidelines, and I believe it’s clear now that almost no one needs annual
bone mineral density testing. I expect the recommendation will be every two
years. In addition, if the baseline test is normal and insurance issues exist, I
believe you can wait longer.
DR CHLEBOWSKI: I’m involved in the process of updating the ASCO bone
health guidelines, and I believe it’s clear now that almost no one needs annual
bone mineral density testing. I expect the recommendation will be every two
years. In addition, if the baseline test is normal and insurance issues exist, I
believe you can wait longer.
As for prophylactic bisphosphonates, the question is, where do you draw the line? Some clinicians might choose to initiate bisphosphonates at a T-score of -1.5, based on Coleman’s data, and that’s probably reasonable (Coleman 2006).
EDITOR
Neil Love, MD
INTERVIEWS
Sharon Giordano, MD, MPH
- Select publications
Rowan T Chlebowski, MD, PhD
- Select publications
Tumor Panel Case Discussion
- Select publications
INTERVIEWS (continued)
Jack Cuzick, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity