|
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you talk about the development of the Oncotype DX assay?
DR LOVE: Can you talk about the development of the Oncotype DX assay?
![]() DR VOGEL: Using archival tissue blocks from past trials, Genomic Health and
Dr Soon Paik from the NSABP (Paik 2004) analyzed about 200 genes that were
reported to possibly relate to outcome in breast cancer. They narrowed that set
down to just 16 genes that could be sorted into logical groups based on the estrogen
receptor, the HER2 protein and proliferation and invasion characteristics of the cells.
DR VOGEL: Using archival tissue blocks from past trials, Genomic Health and
Dr Soon Paik from the NSABP (Paik 2004) analyzed about 200 genes that were
reported to possibly relate to outcome in breast cancer. They narrowed that set
down to just 16 genes that could be sorted into logical groups based on the estrogen
receptor, the HER2 protein and proliferation and invasion characteristics of the cells.
That set of 16 genes plus five reference genes were used to see if breast cancer patients could be sorted into prognostic and predictive groups. When I say “prognostic” I mean to predict the likelihood of recurrence, and when I say “predictive” I mean to predict patients who would benefit from chemotherapy.
So these investigators examined the archival subsets and were able to determine that those 16 genes and five reference genes could be used to sort patients along a continuum they called the recurrence score, which varies from zero to 100. Using simple mathematic regression procedures, that recurrence score could then be translated into a probability of recurrence over 10 years.
![]() DR LOVE: What does the recurrence score tell us?
DR LOVE: What does the recurrence score tell us?
![]() DR VOGEL: The investigators were able to determine that patients who had
low recurrence scores — that is, scores lower than 18 — benefited from
hormonal therapy but derived no additional benefit from the addition of
chemotherapy to their hormonal therapy regimens.
DR VOGEL: The investigators were able to determine that patients who had
low recurrence scores — that is, scores lower than 18 — benefited from
hormonal therapy but derived no additional benefit from the addition of
chemotherapy to their hormonal therapy regimens.
Conversely, patients with high recurrence scores — scores of 31 or higher — showed a clear, statistically significant and large benefit when cytotoxic chemotherapy was added to hormonal therapy — that is, tamoxifen.
In the intermediate group, the group with scores between 18 and 30, no benefit was apparent from the addition of chemotherapy, but the confidence intervals — the statistical certainty of no benefit — were not established.
What came out of that work was the Oncotype DX assay from Genomic Health. It is commercially available and essentially allows selection of patients for hormonal therapy alone or hormonal therapy with chemotherapy in the high-risk group.
![]() DR LOVE: What further studies are being conducted among patients who fall
in the intermediate group?
DR LOVE: What further studies are being conducted among patients who fall
in the intermediate group?
![]() DR VOGEL: In the intermediate-risk group, we’re left with some uncertainty.
An Intergroup clinical trial, known as the TAILORx (2.1) study, is
for patients with ER-positive, node-negative, early-stage — Stage I, small
Stage II — breast cancer. Patients with intermediate recurrence scores will be
randomly assigned to chemotherapy or no chemotherapy, in addition to their
hormonal therapy.
DR VOGEL: In the intermediate-risk group, we’re left with some uncertainty.
An Intergroup clinical trial, known as the TAILORx (2.1) study, is
for patients with ER-positive, node-negative, early-stage — Stage I, small
Stage II — breast cancer. Patients with intermediate recurrence scores will be
randomly assigned to chemotherapy or no chemotherapy, in addition to their
hormonal therapy.
Track 4
![]() DR LOVE: The Oncotype DX data came out before we found out that
adjuvant trastuzumab works so well. At this point, to what extent, if any,
do you think the Oncotype is useful for patients with HER2-positive
tumors?
DR LOVE: The Oncotype DX data came out before we found out that
adjuvant trastuzumab works so well. At this point, to what extent, if any,
do you think the Oncotype is useful for patients with HER2-positive
tumors?
![]() DR VOGEL: That’s an interesting question because some of the data that are
emerging from the Oncotype DX data are perhaps counterintuitive. We can all
cite examples of patients who had HER2-positive disease and were incorporated into the data set for Oncotype DX, yet their recurrence scores were not
necessarily in the high-risk group. That is, the presence of HER2 doesn’t, by
itself, trump all the other genomic prognostic factors. That was a revelation
to some of us because many of us had argued that the presence of the HER2
protein stratifies subsets. All the subsets appeared to indicate a worse prognosis
for patients with HER2 overexpression compared to those whose disease was
HER2-negative.
DR VOGEL: That’s an interesting question because some of the data that are
emerging from the Oncotype DX data are perhaps counterintuitive. We can all
cite examples of patients who had HER2-positive disease and were incorporated into the data set for Oncotype DX, yet their recurrence scores were not
necessarily in the high-risk group. That is, the presence of HER2 doesn’t, by
itself, trump all the other genomic prognostic factors. That was a revelation
to some of us because many of us had argued that the presence of the HER2
protein stratifies subsets. All the subsets appeared to indicate a worse prognosis
for patients with HER2 overexpression compared to those whose disease was
HER2-negative.
The question that remains unanswered by the available data is whether those patients who are HER2 overexpressors and will receive hormonal therapy alone should be receiving trastuzumab. Currently, we don’t have an answer, but I believe trials have been envisioned to answer that question.

Track 9
![]() DR LOVE: The NSABP just launched B-42, evaluating the duration of
adjuvant aromatase inhibitor therapy. What’s your take on the AIs?
DR LOVE: The NSABP just launched B-42, evaluating the duration of
adjuvant aromatase inhibitor therapy. What’s your take on the AIs?
![]() DR VOGEL: When we choose aromatase inhibitor therapy instead of tamoxifen,
we tell patients that, compared to tamoxifen, aromatase inhibitors have a better
safety profile. Fewer thromboembolic and uterine events are associated with their use. We do have the issue of the bone events with the aromatase inhibitors.
DR VOGEL: When we choose aromatase inhibitor therapy instead of tamoxifen,
we tell patients that, compared to tamoxifen, aromatase inhibitors have a better
safety profile. Fewer thromboembolic and uterine events are associated with their use. We do have the issue of the bone events with the aromatase inhibitors.
Then we turn our attention to both the myalgias and arthralgias so patients are aware of those side effects when we start their therapy. Then we talk specifically about the bone data.
My impression of the bone data from the completed and reported adjuvant therapy trials is that, although it was known that the aromatase inhibitors could affect bone density and osteoporotic fractures, those adjuvant trials did not include a systematic, repeated search for bone loss or an attempt to treat that loss with calcium and bisphosphonates.
We tell all our patients that they should have baseline DEXA scans, and our intention will be to repeat their DEXA scans every 24 months, which is the recommendation of our osteoporosis experts. Then, if we see T-scores on the DEXA scans that are more severe than a minus two, we initiate therapy with bisphosphonates.
![]() DR LOVE: Do you use oral or IV bisphosphonates?
DR LOVE: Do you use oral or IV bisphosphonates?
![]() DR VOGEL: Our strategy has been to offer oral bisphosphonates. For about
half the patients, we also have been successful in getting their insurance payers
to pay for IV bisphosphonates — that is, zoledronic acid.
DR VOGEL: Our strategy has been to offer oral bisphosphonates. For about
half the patients, we also have been successful in getting their insurance payers
to pay for IV bisphosphonates — that is, zoledronic acid.
Many patients like zoledronic acid because they don’t have to take a weekly or a daily oral bisphosphonate. They don’t have to endure the GI side effects that occur with oral bisphosphonates. When we can get it paid for, IV bisphosphonates have been well received by patients.
We also have some data from our practice with Adam Brufsky’s Z-fast study (Brufsky 2006) showing that the initiation of zoledronic acid at the start of aromatase inhibitor therapy can prevent bone loss.
The data are not mature enough for fractures. So for us, the bone issue is one we talk about, but it doesn’t dissuade us from using aromatase inhibitors, even in our elderly population, 65 and older, who are probably the majority of the users of the aromatase inhibitors.
Track 10
![]() DR LOVE: How do you approach the patients who have received five
years of an adjuvant aromatase inhibitor?
DR LOVE: How do you approach the patients who have received five
years of an adjuvant aromatase inhibitor?
![]() DR VOGEL: That’s a challenging question.
DR VOGEL: That’s a challenging question.
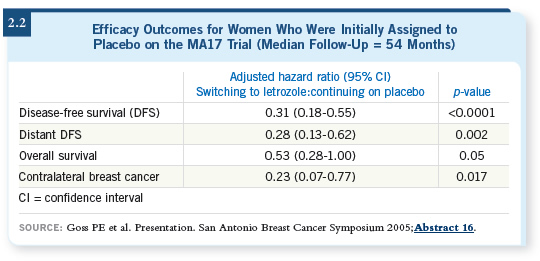
Up until the 2005 San Antonio meeting, I wasn’t certain what the answer was to that question. But I was heartened by the data that were presented, both by Paul Goss and Jim Ingle, on the continued follow-up of the MA17 trial patients and, particularly, those patients who had initially been assigned to placebo and then crossed over to letrozole (Goss 2005).
Two patterns were evident from those data. The first was that the longer a patient received the aromatase inhibitor following five years of tamoxifen, the greater the benefit. It is rare in medical oncology to see a benefit that increases as the duration of therapy increases. But it was clear that the longer the duration of therapy with letrozole was, the greater the benefit was.
Comparing two years to four years, the benefit almost doubled. So for our patients at high risk, especially those with larger tumors and those with positive nodes, based on those data, we’re now telling them they should continue to take their aromatase inhibitor because we know they’re at risk for a very long time — two decades or longer — for recurrence, and these data now show that longer therapy may improve their outcomes.
The other question those data helped us answer relates to patients who have a gap between the end of their tamoxifen therapy and the initiation of their aromatase inhibitor therapy.
The patients who were initially assigned to placebo after five years of tamoxifen in the MA17 trial crossed over to letrozole. Approximately 1,600 patients made the crossover, and their average duration off therapy — that is, the time between the end of their tamoxifen and the initiation of their letrozole — was about 30 months.
Even with that delay in the initiation of the aromatase inhibitor, a statistically significant benefit was demonstrated with the so-called delayed initiation of the aromatase inhibitor after tamoxifen (2.2).
Track 11
![]() DR LOVE: How do you think aromatase inhibitors will “stack up” in the
prevention setting?
DR LOVE: How do you think aromatase inhibitors will “stack up” in the
prevention setting?
![]() DR VOGEL: We’ve all been gratified by the reduced incidence in the adjuvant
trials of second, contralateral breast primary tumors in patients receiving aromatase
inhibitors compared to those receiving tamoxifen.
DR VOGEL: We’ve all been gratified by the reduced incidence in the adjuvant
trials of second, contralateral breast primary tumors in patients receiving aromatase
inhibitors compared to those receiving tamoxifen.
We know from 20 years of tamoxifen data that the reduction of the contralateral second primaries is about 50 percent (Horton 1996), but it’s now evident that the aromatase inhibitors, compared to tamoxifen, bring an additional 20 or 30 percent reduction in the incidence of contralateral second tumors.
This, with preclinical data that show aromatase inhibitors effectively prevent the emergence of invasive breast cancers in animal models, has led to the emergence of two ongoing trials and a soon-to-be-started trial.
The IBIS-II prevention trial in the United Kingdom is comparing anastrozole to placebo. A North American trial compares exemestane to placebo — the so-called MAP.3 study — and the NSABP is proposing a third aromatase inhibitor primary prevention trial — the P-4 trial — which will compare the winner, if you will, of the STAR trial (Wickerham 2006), raloxifene, to letrozole.
Tracks 13-14
![]() DR LOVE: Can you discuss the initial findings from the STAR trial?
DR LOVE: Can you discuss the initial findings from the STAR trial?
![]() DR VOGEL: Between 1999 and 2004 we enrolled 19,747 postmenopausal
patients who were at high risk to the STAR trial (Wickerham 2006). Half of
them received tamoxifen and half received raloxifene.
DR VOGEL: Between 1999 and 2004 we enrolled 19,747 postmenopausal
patients who were at high risk to the STAR trial (Wickerham 2006). Half of
them received tamoxifen and half received raloxifene.
The trial was monitored by a Data and Safety Monitoring Committee, which, in December of 2005, declared that the trial had reached its prestated number of invasive breast cancer events, 327, and so the trial was unblinded.
At the time of the unblinding the occurrence of invasive breast cancer incidents was essentially the same, comparing tamoxifen to raloxifene. The trial recorded 163 invasive breast cancer cases in the tamoxifen arm and 168 in the raloxifene arm. So there was no statistically significant difference.
Compared to the predicted number of invasive breast cancer events at the start of the trial, using the Gail model, the risk reduction was about 50 percent, so the effect of raloxifene was equal to that of tamoxifen. The effect of tamoxifen in the STAR trial was similar to what we had seen in the first breast cancer prevention trial (Fisher 1998).
It is interesting and perhaps surprising that raloxifene did not show as great an effect on the incidence of noninvasive breast cancer. With tamoxifen we saw 57 cases of in situ cancer — which included both ductal carcinoma in situ and lobular carcinoma in situ — and we saw 80 cases with raloxifene. Now, it was not statistically significant, but it did represent 40 percent more in situ cases with raloxifene compared to tamoxifen.
![]() DR LOVE: Can you discuss the safety data from the STAR trial?
DR LOVE: Can you discuss the safety data from the STAR trial?
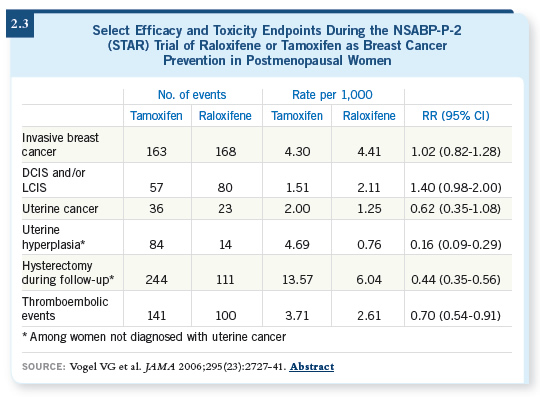
![]() DR VOGEL: We saw approximately a 38 percent reduction in the incidence of
invasive uterine cancer, comparing raloxifene to tamoxifen. Only 23 invasive
uterine events were recorded (2.3) with raloxifene compared to 36 with
tamoxifen. We saw a 50 percent reduction in the number of patients who required hysterectomy with raloxifene compared to tamoxifen. Overall, far
fewer uterine events occurred with raloxifene compared to tamoxifen.
DR VOGEL: We saw approximately a 38 percent reduction in the incidence of
invasive uterine cancer, comparing raloxifene to tamoxifen. Only 23 invasive
uterine events were recorded (2.3) with raloxifene compared to 36 with
tamoxifen. We saw a 50 percent reduction in the number of patients who required hysterectomy with raloxifene compared to tamoxifen. Overall, far
fewer uterine events occurred with raloxifene compared to tamoxifen.
Another major benefit with raloxifene was the rate of serious thromboembolic events (2.3) — deep-vein thrombosis and pulmonary emboli. Thirty percent fewer thrombotic events occurred with raloxifene compared to tamoxifen and also fewer cataracts.
The number of fracture events was about the same for each drug, and both tamoxifen and raloxifene are known to reduce fractures compared to placebo. The number of cardiac events was the same.
Overall, when we looked at the entire data set, it appeared to us that the benefit in terms of reducing the risk of invasive cancer was the same for both drugs. But a substantial improvement in toxicity, both for uterine events and for thromboembolic events, appeared with raloxifene compared to tamoxifen.
![]() DR LOVE: If a patient were to ask you if taking raloxifene would increase her
baseline risk of endometrial cancer or deep vein thrombosis, how would you
respond?
DR LOVE: If a patient were to ask you if taking raloxifene would increase her
baseline risk of endometrial cancer or deep vein thrombosis, how would you
respond?
![]() DR VOGEL: No increased risk of uterine events is apparent using raloxifene. For
clotting events, the risk is increased with raloxifene, but the amount of increase
will be substantially less than what we would expect to see with tamoxifen.
DR VOGEL: No increased risk of uterine events is apparent using raloxifene. For
clotting events, the risk is increased with raloxifene, but the amount of increase
will be substantially less than what we would expect to see with tamoxifen.
![]() DR LOVE: If you don’t believe there’s an increased risk of endometrial cancer,
you’re indirectly comparing raloxifene to the placebo.
DR LOVE: If you don’t believe there’s an increased risk of endometrial cancer,
you’re indirectly comparing raloxifene to the placebo.
![]() DR VOGEL: Yes, and one of the criticisms that has been leveled at the STAR trial is that it had no placebo arm, but we didn’t believe it was ethical to
conduct this trial with a placebo. So we’re left with inferences between the two
treatment arms in STAR and the placebo arms in other trials. When you put
all those data together, it doesn’t appear that raloxifene has a uterine effect.
DR VOGEL: Yes, and one of the criticisms that has been leveled at the STAR trial is that it had no placebo arm, but we didn’t believe it was ethical to
conduct this trial with a placebo. So we’re left with inferences between the two
treatment arms in STAR and the placebo arms in other trials. When you put
all those data together, it doesn’t appear that raloxifene has a uterine effect.