
 |
||||||||

| Tracks 1-28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Can you talk about the discussions that went on between you
and the patient?
DR LOVE: Can you talk about the discussions that went on between you
and the patient?
![]() DR PEGRAM: In this situation, whether or not to use adjuvant systemic
chemotherapy is always a dilemma. That’s always the issue with small,
mammographically detected, lymph node-negative tumors.
DR PEGRAM: In this situation, whether or not to use adjuvant systemic
chemotherapy is always a dilemma. That’s always the issue with small,
mammographically detected, lymph node-negative tumors.
We had serious discussions about chemotherapy and its side effects and to what degree it would reduce the risk of relapse according to computer algorithms such as Adjuvant! Online, which don’t yet incorporate HER2 into the equation.
If you note the hazard ratios for tamoxifen therapy for a small, ER-positive tumor and then add in chemotherapy, you see that it adds very little in terms of percentage differences in these types of estimations. So patients who can view those data critically will often decline chemotherapy for small, nodenegative tumors. This tumor was 1.5 centimeters, so she was a candidate for chemotherapy. However, in the end she declined it.
![]() DR LOVE: You mentioned Adjuvant! Online, which doesn’t currently incorporate HER2 as a prognostic factor or trastuzumab. Did you discuss with her
what you thought her numbers were?
DR LOVE: You mentioned Adjuvant! Online, which doesn’t currently incorporate HER2 as a prognostic factor or trastuzumab. Did you discuss with her
what you thought her numbers were?
![]() DR PEGRAM: Absolutely. In many situations I’ll print out the results from
Adjuvant! Online and thoroughly discuss them with a patient. Because I give
a number of second opinions in a university-based clinic, I find that generally
patients are given what I consider to be overestimates of the utility of systemic
adjuvant chemotherapy for small, lymph node-negative tumors.
DR PEGRAM: Absolutely. In many situations I’ll print out the results from
Adjuvant! Online and thoroughly discuss them with a patient. Because I give
a number of second opinions in a university-based clinic, I find that generally
patients are given what I consider to be overestimates of the utility of systemic
adjuvant chemotherapy for small, lymph node-negative tumors.
When they see the real numbers, it is sometimes sobering, but I believe it empowers patients to make informed decisions. So I find these types of algorithms useful.
Tracks 6-7
![]() DR LOVE: What was your estimate of how the risk of relapse would
have been affected if this patient had been willing to go “full bore” with
endocrine therapy, chemotherapy and trastuzumab?
DR LOVE: What was your estimate of how the risk of relapse would
have been affected if this patient had been willing to go “full bore” with
endocrine therapy, chemotherapy and trastuzumab?
![]() DR PEGRAM: The hazard ratios for all the adjuvant trastuzumab trials that have
been reported — all of which have used chemotherapy in combination with
trastuzumab or chemotherapy followed by trastuzumab — are coming in at
around a half, with remarkable consistency across the studies. Remember, that
hazard ratio of 0.5 is above and beyond chemotherapy and hormone therapy.
DR PEGRAM: The hazard ratios for all the adjuvant trastuzumab trials that have
been reported — all of which have used chemotherapy in combination with
trastuzumab or chemotherapy followed by trastuzumab — are coming in at
around a half, with remarkable consistency across the studies. Remember, that
hazard ratio of 0.5 is above and beyond chemotherapy and hormone therapy.
In subset analyses, the hazard ratio in favor of trastuzumab is similar for ER-positive and ER-negative disease, and in the European HERA trial (Piccart- Gebhart 2005), it was similar for lymph node-negative and node-positive disease. So trastuzumab will be the workhorse for a patient like this in the modern era. Chemotherapy and endocrine therapy will have a much less robust effect compared to trastuzumab.
She felt comfortable that the efficacy of trastuzumab, which had recently been demonstrated, would be sufficient in her mind without chemotherapy to reduce her risk of recurrence when administered in combination with endocrine therapy.
If I were to have administered chemotherapy, I would have offered her a nonanthracycline-based regimen as one of the options, based on the BCIRG data, specifically TCH (Slamon 2005).
Track 10
![]() DR LOVE: What about endocrine therapy for this patient? When you look
at tamoxifen, the aromatase inhibitors and fulvestrant, which seems to
make the most sense in terms of combining with trastuzumab?
DR LOVE: What about endocrine therapy for this patient? When you look
at tamoxifen, the aromatase inhibitors and fulvestrant, which seems to
make the most sense in terms of combining with trastuzumab?
![]() DR PEGRAM: Fulvestrant makes the most sense because in HER2-positive breast
tumor cells there is ligand-independent activation of the estrogen receptor. That
is, the cross talk between HER2 signaling and the estrogen receptor can activate estrogen-dependent genes in the absence of estradiol. That predicts an absence of
estradiol with aromatase inhibitors — no ligand for the ER — but the ER can
still be turned on by HER2 signaling. So that’s a strike against aromatase inhibitors.
Tamoxifen can also be more agonistic as a result of this cross talk mechanism.
DR PEGRAM: Fulvestrant makes the most sense because in HER2-positive breast
tumor cells there is ligand-independent activation of the estrogen receptor. That
is, the cross talk between HER2 signaling and the estrogen receptor can activate estrogen-dependent genes in the absence of estradiol. That predicts an absence of
estradiol with aromatase inhibitors — no ligand for the ER — but the ER can
still be turned on by HER2 signaling. So that’s a strike against aromatase inhibitors.
Tamoxifen can also be more agonistic as a result of this cross talk mechanism.
The question is, how can you tackle such a complex issue? It would be ideal to eliminate the estrogen receptor, and that’s exactly what fulvestrant does. Therefore, it is appealing from a theoretical point of view to incorporate HER2-directed therapy with fulvestrant, and we have a randomized Phase II trial under way in the metastatic setting comparing fulvestrant alone to trastuzumab alone to the combination. It’s accruing slowly, unfortunately, and may have to be pared down to get some point estimate on the activity of the combination in the future.
![]() DR LOVE: When you see a postmenopausal patient with metastatic disease
that’s ER-positive and HER2-positive, do you use trastuzumab with hormonal
therapy?
DR LOVE: When you see a postmenopausal patient with metastatic disease
that’s ER-positive and HER2-positive, do you use trastuzumab with hormonal
therapy?
![]() DR PEGRAM: Absolutely. I have a number of patients on fulvestrant and
trastuzumab who are doing well, although they were started on the treatment
off protocol because our protocol wasn’t open when they started. I’ve had
some nice anecdotal responders on that combination. Remember that many of
these patients have already received adjuvant aromatase inhibitors anyway. So
fulvestrant is a reasonable consideration when they relapse.
DR PEGRAM: Absolutely. I have a number of patients on fulvestrant and
trastuzumab who are doing well, although they were started on the treatment
off protocol because our protocol wasn’t open when they started. I’ve had
some nice anecdotal responders on that combination. Remember that many of
these patients have already received adjuvant aromatase inhibitors anyway. So
fulvestrant is a reasonable consideration when they relapse.
Tracks 16-17
![]() DR LOVE: If your patient’s disease had been multiple node-positive and
she had no special concerns about chemotherapy, which chemotherapy
would you have used?
DR LOVE: If your patient’s disease had been multiple node-positive and
she had no special concerns about chemotherapy, which chemotherapy
would you have used?
![]() DR PEGRAM: It all depends on one’s estimate of the cardiac risk. If it was for
a healthy patient who had a lot of positive nodes and I thought that she could
safely tolerate an anthracycline-based regimen, then I would consider it.
DR PEGRAM: It all depends on one’s estimate of the cardiac risk. If it was for
a healthy patient who had a lot of positive nodes and I thought that she could
safely tolerate an anthracycline-based regimen, then I would consider it.
The lion’s share of young, healthy patients will tolerate anthracycline-based regimens, and even in the BCIRG 006 cohort, the numerically — though not statistically — superior arm is clearly in favor of the anthracycline followed by docetaxel/trastuzumab regimen.
![]() DR LOVE: What are your thoughts on the TOPO II data that came out of that
trial?
DR LOVE: What are your thoughts on the TOPO II data that came out of that
trial?
![]() DR PEGRAM: We realized, based on the design of BCIRG 006, that we had
a unique opportunity because we had a nonanthracycline arm and an anthracycline
arm, both of which included trastuzumab, in a pure population of
patients with HER2-amplified disease.
DR PEGRAM: We realized, based on the design of BCIRG 006, that we had
a unique opportunity because we had a nonanthracycline arm and an anthracycline
arm, both of which included trastuzumab, in a pure population of
patients with HER2-amplified disease.
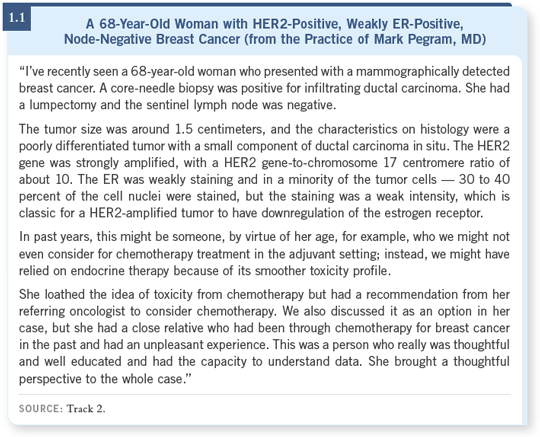
Dennis Slamon presented the preliminary data on the amplification of TOPO II at the plenary session during the 2005 San Antonio meeting, and Mike Press had a poster also summarizing the data (Press 2005; Slamon 2005; [1.2]).
It’s important to realize that it was an interim subset analysis of only the first couple of thousand of the 3,200 patients, and longer follow-up is needed. With those caveats, the hypothesis that coamplification of TOPO II and HER2 does confer additional benefit from anthracyclines seems to be indicated by this preliminary analysis.
Track 19
![]() DR LOVE: For a patient with HER2-negative, node-positive disease, what
tends to be the chemotherapeutic regimen that you use off protocol?
DR LOVE: For a patient with HER2-negative, node-positive disease, what
tends to be the chemotherapeutic regimen that you use off protocol?
![]() DR PEGRAM: It depends on the patient’s age, performance status and
comorbid medical conditions. We have any number of active regimens to
choose from, and I usually give the patients a menu of options (1.3).
DR PEGRAM: It depends on the patient’s age, performance status and
comorbid medical conditions. We have any number of active regimens to
choose from, and I usually give the patients a menu of options (1.3).
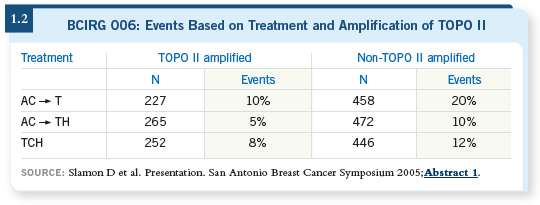
When I see patients in consultation as a second opinion, if someone has been referred to me with node-positive disease and it has been recommended they receive dose-dense adjuvant chemotherapy, TAC or FEC followed by docetaxel, I’d say those are perfectly good regimens for lymph node-positive, early-stage breast cancer.
![]() DR LOVE: What about the controversy over whether TAC is better than dose-dense
chemotherapy for patients with ER-positive disease?
DR LOVE: What about the controversy over whether TAC is better than dose-dense
chemotherapy for patients with ER-positive disease?
![]() DR PEGRAM: For ER-positive disease, I have a hard time justifying the
dose-dense approach. Findings for that subset, which is fully two thirds of the
N9741 cohort, are negative to date (Citron 2003; Hudis 2005).
DR PEGRAM: For ER-positive disease, I have a hard time justifying the
dose-dense approach. Findings for that subset, which is fully two thirds of the
N9741 cohort, are negative to date (Citron 2003; Hudis 2005).
If you look at the principle on which the dose-dense adjuvant regimen was devised — the Norton-Simon hypothesis — you see that substantial regrowth of tumor cell populations between cycles is necessary for the dose-dense approach to work. For an indolent, ER-positive, slow-growing tumor, there will not be a substantial difference in the number of cells in somebody’s body over a one-week period.
The Norton-Simon hypothesis predicts a population of indolent, slow-growing breast tumors, for which dose-dense treatment is not necessary, and that’s exactly what the data set shows.
![]() DR LOVE: So what chemotherapy regimen do you tend to use for those
patients with ER-positive disease?
DR LOVE: So what chemotherapy regimen do you tend to use for those
patients with ER-positive disease?
![]() DR PEGRAM: For ER-positive patients, again, it depends on their age, et
cetera. If they’re getting on in years, I’m more likely to use AC followed by
weekly paclitaxel, for example, because that’s so well tolerated. If they are
young, fit, in their thirties, have no comorbid medical illnesses and have a
number of positive nodes, I would have no hesitation using TAC (Martin
2005) because we participated in some of those TAC trials and we’re comfortable
with the regimen when we use pegfilgrastim.
DR PEGRAM: For ER-positive patients, again, it depends on their age, et
cetera. If they’re getting on in years, I’m more likely to use AC followed by
weekly paclitaxel, for example, because that’s so well tolerated. If they are
young, fit, in their thirties, have no comorbid medical illnesses and have a
number of positive nodes, I would have no hesitation using TAC (Martin
2005) because we participated in some of those TAC trials and we’re comfortable
with the regimen when we use pegfilgrastim.
Track 21
![]() DR LOVE: What were your thoughts about Steve Jones’ presentation at
San Antonio 2005 of the US Oncology adjuvant trial of docetaxel/cyclophosphamide
versus AC ( Jones 2005; [1.4])?
DR LOVE: What were your thoughts about Steve Jones’ presentation at
San Antonio 2005 of the US Oncology adjuvant trial of docetaxel/cyclophosphamide
versus AC ( Jones 2005; [1.4])?
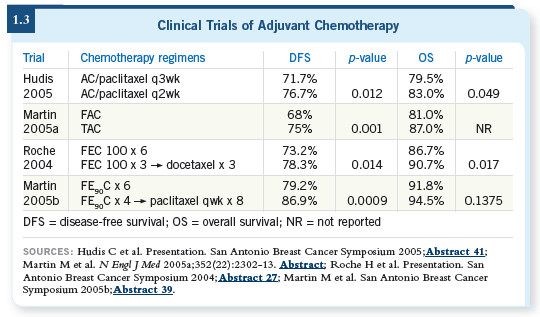
![]() DR PEGRAM: It was an exciting presentation, and I’m not surprised at all by
the data. Steve presented a randomized trial for patients with early-stage breast
cancer, approximately 40 to 50 percent of whom had node-negative disease.
DR PEGRAM: It was an exciting presentation, and I’m not surprised at all by
the data. Steve presented a randomized trial for patients with early-stage breast
cancer, approximately 40 to 50 percent of whom had node-negative disease.
They were randomly assigned to four cycles of AC versus four cycles of TC. They showed a significant relapse-free survival advantage with the TC compared to the AC arm, and a numeric trend even appeared in the survival analysis, although it hasn’t reached statistical significance yet. Steve Jones concluded — and probably rightly so — that this constitutes a new regimen that replaces AC. If you’re going to use a four-cycle regimen, you probably wouldn’t want to use AC anymore, based on this data set.
I was also favorably surprised by the toxicity and safety data. The TC was well tolerated compared to AC. It goes to show that we probably underestimate the toxicity of AC routinely because we’re so used to prescribing it.
I saw a young woman within the past couple of weeks in my clinic with newly diagnosed doxorubicin cardiotoxicity after adjuvant therapy for what will probably be curable breast cancer. It’s sobering and scary when you see cases like this.
Track 23
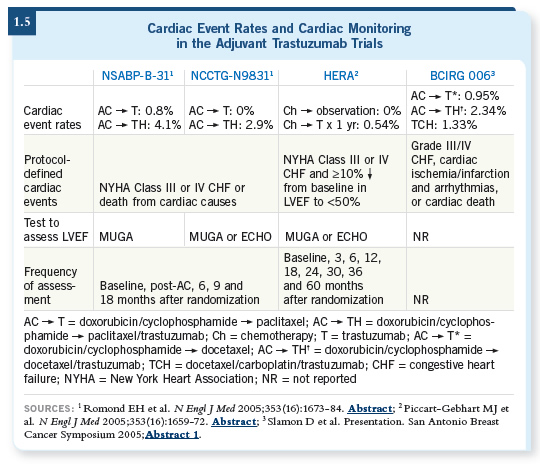
![]() DR LOVE: Let’s talk about the cardiac issues and trastuzumab. It’s difficult
for a physician in practice to sort this out because each trial approached it
differently (1.5).
DR LOVE: Let’s talk about the cardiac issues and trastuzumab. It’s difficult
for a physician in practice to sort this out because each trial approached it
differently (1.5).
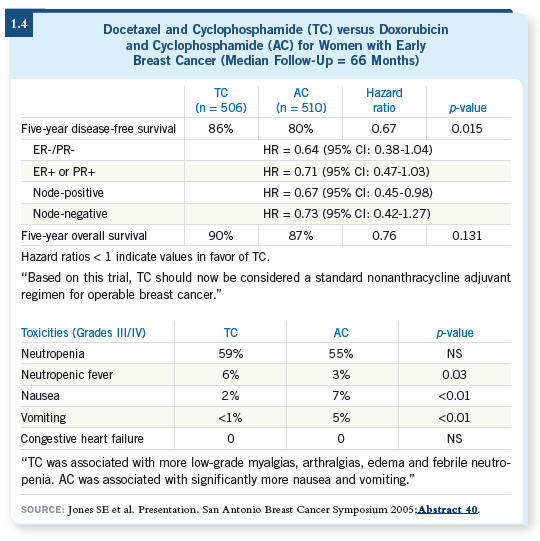
![]() DR PEGRAM: If you’re going to consider an anthracycline-based adjuvant
regimen followed by trastuzumab with taxanes, you need to tell patients that it
carries a defined risk of cardiotoxicity. In particular, in the NSABP-B-31 adjuvant trastuzumab trial, after four cycles of AC approximately four to
five percent of the patients were ineligible for adjuvant trastuzumab at all. If
you were in clinical practice, it would be important to measure the ejection
fraction before and after the AC to make sure that your patient would have
met the eligibility for the study and you could draw on that safety database.
DR PEGRAM: If you’re going to consider an anthracycline-based adjuvant
regimen followed by trastuzumab with taxanes, you need to tell patients that it
carries a defined risk of cardiotoxicity. In particular, in the NSABP-B-31 adjuvant trastuzumab trial, after four cycles of AC approximately four to
five percent of the patients were ineligible for adjuvant trastuzumab at all. If
you were in clinical practice, it would be important to measure the ejection
fraction before and after the AC to make sure that your patient would have
met the eligibility for the study and you could draw on that safety database.
Moreover, during the year of adjuvant trastuzumab for the patients who received the drug, an additional approximately 15 percent of the patients had to drop out because of decreases in ejection fraction, which I find alarming. My fear is that in the community, busy practitioners will forget to obtain those ECHOs and MUGAs every three months, which was done on all of the adjuvant trastuzumab trials.
I’m fearful of what might happen for patients who have marked decreases in ejection fraction but may not be having symptoms from heart failure yet, and because they didn’t get their ECHO or MUGA they are simply continued on more trastuzumab. That scares me, and clinicians need to know that if they’re going to prescribe adjuvant trastuzumab, they should do so following the same guidelines that were used in those protocols.
![]() DR LOVE: Can you go through exactly what those were?
DR LOVE: Can you go through exactly what those were?
![]() DR PEGRAM: It was an ejection fraction assessment every three months
during the one year of trastuzumab. If the ejection fraction decreased to less than institutional norms, patients had to drop out. If it dropped 15 points
and was above institutional norms, they had to hold the trastuzumab, at least
temporarily, and wait for recovery. If recovery was evident on a follow-up one
month later, then they were allowed to attempt to reinstitute it, as long as they
were not symptomatic or at lower than institutional norms. These protocol
guidelines are available, and they should be strictly followed if you’re going to
use anthracyclines.
DR PEGRAM: It was an ejection fraction assessment every three months
during the one year of trastuzumab. If the ejection fraction decreased to less than institutional norms, patients had to drop out. If it dropped 15 points
and was above institutional norms, they had to hold the trastuzumab, at least
temporarily, and wait for recovery. If recovery was evident on a follow-up one
month later, then they were allowed to attempt to reinstitute it, as long as they
were not symptomatic or at lower than institutional norms. These protocol
guidelines are available, and they should be strictly followed if you’re going to
use anthracyclines.
Track 25
![]() DR LOVE: I heard that the NSABP and BCIRG are interested in the
concept of an adjuvant trial evaluating bevacizumab and trastuzumab. Do
you think that will happen?
DR LOVE: I heard that the NSABP and BCIRG are interested in the
concept of an adjuvant trial evaluating bevacizumab and trastuzumab. Do
you think that will happen?
![]() DR PEGRAM: I believe it will, but it all hinges on the pilot adjuvant bevacizumab
trial that’s under way now through ECOG. So we’re anxiously
awaiting the safety analysis of that trial. Of course, the primary endpoint for
that study is cardiac safety.
DR PEGRAM: I believe it will, but it all hinges on the pilot adjuvant bevacizumab
trial that’s under way now through ECOG. So we’re anxiously
awaiting the safety analysis of that trial. Of course, the primary endpoint for
that study is cardiac safety.
Practicing clinicians should probably wait on the sidelines to see these safety data sets before embarking on any of these combinations on their own. These types of combinations are of serious concern, and clinicians shouldn’t do anything off protocol in the absence of the Phase II data.