|
||||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
EDITOR’S NOTE: This interview focuses on a recent survey of 12 clinical investigators for a recent Breast Cancer Think Tank. For more information, go to BreastCancerUpdate.com/thinktank
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you talk about the use of sequential tamoxifen/aromatase
inhibitors versus up-front aromatase inhibitors in the adjuvant setting?
DR LOVE: Can you talk about the use of sequential tamoxifen/aromatase
inhibitors versus up-front aromatase inhibitors in the adjuvant setting?
![]() DR CARLSON: The different methods of using aromatase inhibitors or incorporating
them — initial aromatase inhibitor therapy versus sequential after two
to three years of tamoxifen versus extended after five years — have never truly
been studied in a randomized fashion, one against another. The BIG 1-98 trial
(Thürlimann 2005) will give us the first look at that sort of comparison.
DR CARLSON: The different methods of using aromatase inhibitors or incorporating
them — initial aromatase inhibitor therapy versus sequential after two
to three years of tamoxifen versus extended after five years — have never truly
been studied in a randomized fashion, one against another. The BIG 1-98 trial
(Thürlimann 2005) will give us the first look at that sort of comparison.
The real question is whether tamoxifen does something to prime the breast cancer cells and cause the aromatase inhibitor to be more effective. Or, rather, is it that the population of women and the characteristics of their breast cancer change over time in a way that would make the aromatase inhibitors — or any hormonal therapy — more effective?
I believe a substantial amount of data exists to support the selection bias theory that the population of breast cancer patients over time is changing. You would expect the endocrine-resistant, receptor-positive breast cancer to recur earlier, so those women are removed from the denominator.
If you have a sensitive population and an insensitive population with hormone receptor-positive tumors — even with no difference in efficacy between the hormonal therapies — you should expect to see an increasing effect the later in time you initiate the therapy. However, it’s hard to have a drug that’s so effective down the road that you are able to regain the loss of two to three absolute percentage points that women may experience when the drug is used in this context.
![]() DR LOVE: If you were to treat 100 postmenopausal women, what prescription
would they likely receive before leaving your office?
DR LOVE: If you were to treat 100 postmenopausal women, what prescription
would they likely receive before leaving your office?
![]() DR CARLSON: The vast majority would walk out with a prescription for an
aromatase inhibitor — usually anastrozole in my practice. We have to establish
a practice pattern, and mine is to lead with an aromatase inhibitor. It is
interesting how expert panels interpreted the emerging aromatase inhibitor
data differently. Within 10 to 14 days of the initial ATAC presentation, the
NCCN panel had modified the guidelines to allow anastrozole as an alternative
to tamoxifen as initial hormonal therapy for postmenopausal patients with
ER-positive disease.
DR CARLSON: The vast majority would walk out with a prescription for an
aromatase inhibitor — usually anastrozole in my practice. We have to establish
a practice pattern, and mine is to lead with an aromatase inhibitor. It is
interesting how expert panels interpreted the emerging aromatase inhibitor
data differently. Within 10 to 14 days of the initial ATAC presentation, the
NCCN panel had modified the guidelines to allow anastrozole as an alternative
to tamoxifen as initial hormonal therapy for postmenopausal patients with
ER-positive disease.
The ASCO panel initially believed that tamoxifen should remain the standard hormonal therapy, but that guideline, over time, has also changed. Currently, the NCCN and the ASCO guidelines are essentially identical in terms of up-front hormonal therapy.
Track 5
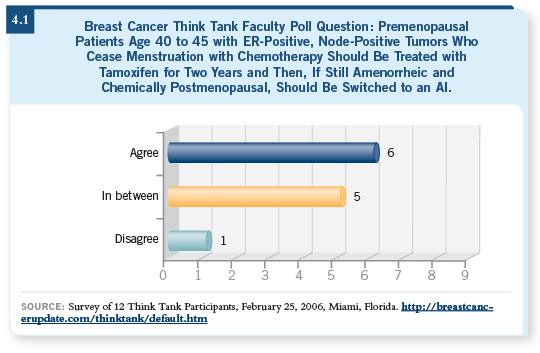
![]() DR LOVE: Do you agree or disagree (4.1): “Premenopausal patients aged
40 to 45 with ER-positive, node-positive tumors who cease menstruation
with chemotherapy should be treated with tamoxifen for two years
and then, if still amenorrheic and chemically postmenopausal, should be
switched to an aromatase inhibitor.”
DR LOVE: Do you agree or disagree (4.1): “Premenopausal patients aged
40 to 45 with ER-positive, node-positive tumors who cease menstruation
with chemotherapy should be treated with tamoxifen for two years
and then, if still amenorrheic and chemically postmenopausal, should be
switched to an aromatase inhibitor.”
![]() DR CARLSON: I would feel comfortable switching a woman in that situation
to an aromatase inhibitor based on the trial data that we have. The difficulty
with that statement, of course, is that the crossover trials, the switching trials,
did not include such women. The women had to be postmenopausal at the
time of diagnosis. So one issue is how biologically similar we think women
are who have gone through chemically induced menopause to those who are
naturally postmenopausal at the time of diagnosis.
DR CARLSON: I would feel comfortable switching a woman in that situation
to an aromatase inhibitor based on the trial data that we have. The difficulty
with that statement, of course, is that the crossover trials, the switching trials,
did not include such women. The women had to be postmenopausal at the
time of diagnosis. So one issue is how biologically similar we think women
are who have gone through chemically induced menopause to those who are
naturally postmenopausal at the time of diagnosis.
![]() DR LOVE: Do you usually switch such patients to an aromatase inhibitor?
DR LOVE: Do you usually switch such patients to an aromatase inhibitor?
![]() DR CARLSON: It is a strategy that I have used. More commonly, I tend to
administer a full five years of tamoxifen and then cross over to letrozole, as
in the MA17 trial (Goss 2005). The MA17 trial eligibility criteria did allow
women who had become postmenopausal during the five years of tamoxifen.
DR CARLSON: It is a strategy that I have used. More commonly, I tend to
administer a full five years of tamoxifen and then cross over to letrozole, as
in the MA17 trial (Goss 2005). The MA17 trial eligibility criteria did allow
women who had become postmenopausal during the five years of tamoxifen.
![]() DR LOVE: What about a patient with 10 positive nodes? Would you still keep
the tamoxifen going for five years?
DR LOVE: What about a patient with 10 positive nodes? Would you still keep
the tamoxifen going for five years?
![]() DR CARLSON: The higher the risk for recurrence, the more willing I would
be to consider crossover to an aromatase inhibitor earlier. That’s not necessarily
logical because my confidence level doesn’t increase in that situation.
DR CARLSON: The higher the risk for recurrence, the more willing I would
be to consider crossover to an aromatase inhibitor earlier. That’s not necessarily
logical because my confidence level doesn’t increase in that situation.
![]() DR LOVE: Obviously the concern is that if the woman were to start menstruating
again, you’d then have an ineffective therapy. The other option is, at
some point, even at the beginning, to include an LHRH agonist or remove
the ovaries — even if the woman has stopped menstruating — just to be sure.
DR LOVE: Obviously the concern is that if the woman were to start menstruating
again, you’d then have an ineffective therapy. The other option is, at
some point, even at the beginning, to include an LHRH agonist or remove
the ovaries — even if the woman has stopped menstruating — just to be sure.
![]() DR CARLSON: That’s an option. The important point, however, that you’re
raising indirectly is that of the women who you believe have become
postmenopausal, secondary to adjuvant chemotherapy, many will experience a resumption of ovarian function. In that context, if you’re going to use
an aromatase inhibitor, you must be confident not only that the woman is
postmenopausal when you start it but also that she remains so as the treatment
is continued.
DR CARLSON: That’s an option. The important point, however, that you’re
raising indirectly is that of the women who you believe have become
postmenopausal, secondary to adjuvant chemotherapy, many will experience a resumption of ovarian function. In that context, if you’re going to use
an aromatase inhibitor, you must be confident not only that the woman is
postmenopausal when you start it but also that she remains so as the treatment
is continued.
Track 7
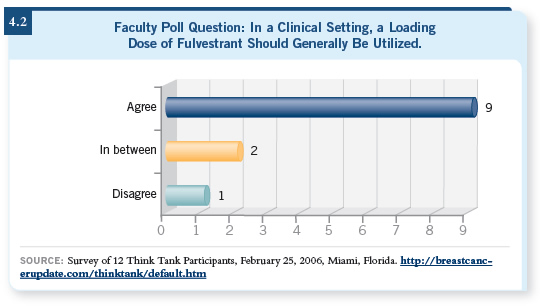
![]() DR LOVE: Do you agree or disagree with the following statement: “In a
clinical setting, a loading dose of fulvestrant generally should be used.”
DR LOVE: Do you agree or disagree with the following statement: “In a
clinical setting, a loading dose of fulvestrant generally should be used.”
![]() DR CARLSON: I agree.
DR CARLSON: I agree.
![]() DR LOVE: Is that something you do in your practice?
DR LOVE: Is that something you do in your practice?
![]() DR CARLSON: Yes, it is.
DR CARLSON: Yes, it is.
![]() DR LOVE: We’re seeing a lot of that from both investigators and oncologists in
practice (4.2). Where do you think we are heading with fulvestrant in terms
of dose and schedule and use for premenopausal women?
DR LOVE: We’re seeing a lot of that from both investigators and oncologists in
practice (4.2). Where do you think we are heading with fulvestrant in terms
of dose and schedule and use for premenopausal women?
![]() DR CARLSON: I continue to see an increase in the number of patients treated
with fulvestrant. That’s reasonable, and experience has confirmed the tolerability
of the drug and the efficacy of the therapy. My expectation is we’ll see
nothing but increased use of fulvestrant. In terms of use for the premenopausal
woman, I believe that in the metastatic setting, we will see increasing numbers
of patients treated with fulvestrant after they are put in a menopausal state. In
part this is because I believe the truly limited number of endocrine agents we
have available for the treatment of premenopausal breast cancer means that,
functionally, after a premenopausal woman has been treated with tamoxifen,
you’re obligated to make her postmenopausal.
DR CARLSON: I continue to see an increase in the number of patients treated
with fulvestrant. That’s reasonable, and experience has confirmed the tolerability
of the drug and the efficacy of the therapy. My expectation is we’ll see
nothing but increased use of fulvestrant. In terms of use for the premenopausal
woman, I believe that in the metastatic setting, we will see increasing numbers
of patients treated with fulvestrant after they are put in a menopausal state. In
part this is because I believe the truly limited number of endocrine agents we
have available for the treatment of premenopausal breast cancer means that,
functionally, after a premenopausal woman has been treated with tamoxifen,
you’re obligated to make her postmenopausal.
Once she’s postmenopausal, the whole spectrum of endocrine agents, which are effective in the postmenopausal woman, become available.
![]() DR LOVE: Do you have patients who are on an LHRH agonist and fulvestrant?
DR LOVE: Do you have patients who are on an LHRH agonist and fulvestrant?
![]() DR CARLSON: In the metastatic setting. Because my expectation is that the
women will be on hormone therapy for some length of time, I often send
those women to the gynecologic oncologist for a laparoscopic oophorectomy.
DR CARLSON: In the metastatic setting. Because my expectation is that the
women will be on hormone therapy for some length of time, I often send
those women to the gynecologic oncologist for a laparoscopic oophorectomy.
Track 11
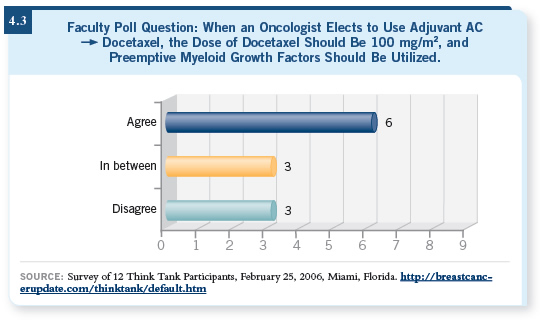
![]() DR LOVE: Here is another Think Tank poll question (4.3). “Putting
cost and reimbursement issues aside, do you agree or disagree that if an
oncologist elects to use adjuvant AC followed by docetaxel, the dose
of docetaxel should be 100 mg/m2 — every three weeks — and that
preemptive myeloid growth factor should be used?”
DR LOVE: Here is another Think Tank poll question (4.3). “Putting
cost and reimbursement issues aside, do you agree or disagree that if an
oncologist elects to use adjuvant AC followed by docetaxel, the dose
of docetaxel should be 100 mg/m2 — every three weeks — and that
preemptive myeloid growth factor should be used?”
![]() DR CARLSON: Docetaxel administered every three weeks at 100 mg/m2 is
a reasonable taxane to use following AC chemotherapy. I have no difficulty
with that. ECOG trial E1199 suggested equal efficacy to paclitaxel in that
setting (Sparano 2005). Perhaps a little more toxicity, especially febrile neutropenia,
occurred with the every three-week regimen. Given the increased
frequency of febrile neutropenia, growth factors would be reasonable to use
with that dose and schedule.
DR CARLSON: Docetaxel administered every three weeks at 100 mg/m2 is
a reasonable taxane to use following AC chemotherapy. I have no difficulty
with that. ECOG trial E1199 suggested equal efficacy to paclitaxel in that
setting (Sparano 2005). Perhaps a little more toxicity, especially febrile neutropenia,
occurred with the every three-week regimen. Given the increased
frequency of febrile neutropenia, growth factors would be reasonable to use
with that dose and schedule.
![]() DR LOVE: Gary Lyman has data suggesting a surprising lack of use of preemptive
growth factors in the adjuvant setting (Lyman 2003). I thought everyone
knew you had to give growth factors when you use TAC. According to him,
a significant number of patients are being treated with adjuvant TAC without
growth factors. Any take on what’s going on?
DR LOVE: Gary Lyman has data suggesting a surprising lack of use of preemptive
growth factors in the adjuvant setting (Lyman 2003). I thought everyone
knew you had to give growth factors when you use TAC. According to him,
a significant number of patients are being treated with adjuvant TAC without
growth factors. Any take on what’s going on?
![]() DR CARLSON: I don’t understand that. TAC certainly causes febrile neutropenia
with high enough frequency that growth factors should be used. The
NCCN Breast Cancer Treatment Guideline specifies the use of growth factors with two of the adjuvant chemotherapy regimens. One would be TAC and the
other would be a dose-dense chemotherapy regimen.
DR CARLSON: I don’t understand that. TAC certainly causes febrile neutropenia
with high enough frequency that growth factors should be used. The
NCCN Breast Cancer Treatment Guideline specifies the use of growth factors with two of the adjuvant chemotherapy regimens. One would be TAC and the
other would be a dose-dense chemotherapy regimen.
Track 12
![]() DR LOVE: Do you agree or disagree? “Patients with strongly ER-positive,
PR-positive, node-positive tumors who require adjuvant therapy should
generally receive TAC chemotherapy as opposed to dose-dense AC
DR LOVE: Do you agree or disagree? “Patients with strongly ER-positive,
PR-positive, node-positive tumors who require adjuvant therapy should
generally receive TAC chemotherapy as opposed to dose-dense AC
![]() paclitaxel and other regimens.”
paclitaxel and other regimens.”
![]() DR CARLSON: One of the difficulties in evaluating the adjuvant therapy
studies and making cross-study comparisons is that the patient populations are
often quite different. The doses and schedules of chemotherapy are almost by
definition different.
DR CARLSON: One of the difficulties in evaluating the adjuvant therapy
studies and making cross-study comparisons is that the patient populations are
often quite different. The doses and schedules of chemotherapy are almost by
definition different.
The analyses of dose-dense chemotherapy and TAC in hormone receptor-positive patients are provocative. Dose-dense chemotherapy showed very little benefit in receptor-positive breast cancer, whereas not much difference in efficacy appeared between the patients with ER-negative and ER-positive disease in the TAC study. Those are indirect comparisons, so I’m not sure we can make much of that specific finding. It’ll be interesting to see, as ECOGE1199 unfolds, if a differential responsiveness appears with docetaxel versus paclitaxel based on ER status, because that’s what you’d have to hypothesize.
![]() DR LOVE: Actually, most oncologists and clinical investigators agree with
you, and they weren’t ready to abandon dose-dense AC
DR LOVE: Actually, most oncologists and clinical investigators agree with
you, and they weren’t ready to abandon dose-dense AC![]() paclitaxel, which,
according to our Patterns of Care studies with both investigators and oncologists,
is by far the most common chemotherapeutic regimen being used for
node-positive disease. The last time I spoke with you, that was your chosen
treatment for patients with node-positive disease. Is that the case?
paclitaxel, which,
according to our Patterns of Care studies with both investigators and oncologists,
is by far the most common chemotherapeutic regimen being used for
node-positive disease. The last time I spoke with you, that was your chosen
treatment for patients with node-positive disease. Is that the case?
![]() DR CARLSON: Yes, and it continues to be the case.
DR CARLSON: Yes, and it continues to be the case.
I’ve been surprised at how nontoxic dose-dense AC followed by paclitaxel is to deliver. You can argue it’s even less toxic and easier to deliver than the every three-week regimens. My experience with TAC is that it’s a difficult regimen. It’s a tolerable regimen — women can get through it — but it’s a much more difficult regimen in terms of acute toxicities.
Track 13
![]() DR LOVE: Do you think that every two-week AC without a taxane with
only growth factor support is a reasonable regimen?
DR LOVE: Do you think that every two-week AC without a taxane with
only growth factor support is a reasonable regimen?
![]() DR CARLSON: It’s a reasonable regimen, and I use it for the patients for whom
I do not consider a taxane necessary. It’s based on the belief — and it’s just
a belief, it’s not yet proven — that if dose-dense AC followed by paclitaxel,
or the ATC dose-dense regimen, is superior, it’s likely that every two-week
AC should be superior, or at least equal to every three-week AC. Again, I’m
impressed at how nontoxic it is when you use growth factors. I believe women
like to get through these therapies quickly, and you shorten the duration of
treatment with the dose-dense regimens.
DR CARLSON: It’s a reasonable regimen, and I use it for the patients for whom
I do not consider a taxane necessary. It’s based on the belief — and it’s just
a belief, it’s not yet proven — that if dose-dense AC followed by paclitaxel,
or the ATC dose-dense regimen, is superior, it’s likely that every two-week
AC should be superior, or at least equal to every three-week AC. Again, I’m
impressed at how nontoxic it is when you use growth factors. I believe women
like to get through these therapies quickly, and you shorten the duration of
treatment with the dose-dense regimens.
Track 14
![]() DR LOVE: Do you agree or disagree with the following statement: “For
patients with minimally symptomatic metastatic breast cancer in nonvisceral
sites, the optimal first-line chemotherapy regimen is single-agent
capecitabine.”
DR LOVE: Do you agree or disagree with the following statement: “For
patients with minimally symptomatic metastatic breast cancer in nonvisceral
sites, the optimal first-line chemotherapy regimen is single-agent
capecitabine.”
![]() DR CARLSON: I would agree with that.
DR CARLSON: I would agree with that.
![]() DR LOVE: For patients with metastatic disease, we are seeing a lot more
earlier use of capecitabine by clinical investigators and breast cancer specialists
compared to those in community practice. In general, is capecitabine your
first-line chemotherapeutic agent?
DR LOVE: For patients with metastatic disease, we are seeing a lot more
earlier use of capecitabine by clinical investigators and breast cancer specialists
compared to those in community practice. In general, is capecitabine your
first-line chemotherapeutic agent?
![]() DR CARLSON: Yes, capecitabine has efficacy that is in the ballpark of any
single agent, and I tend to treat metastatic breast cancer that’s not in visceral
crisis with single-agent therapy. The toxicity profile of capecitabine is favorable,
and the women appreciate being able to take an oral medication, not
having to go to the infusion center and not having to come back as frequently.
It’s an agent that, at doses that are typically used, is associated with a predictable
toxicity experience. I use 1,000 mg/m2 twice daily — two weeks out of
three weeks.
DR CARLSON: Yes, capecitabine has efficacy that is in the ballpark of any
single agent, and I tend to treat metastatic breast cancer that’s not in visceral
crisis with single-agent therapy. The toxicity profile of capecitabine is favorable,
and the women appreciate being able to take an oral medication, not
having to go to the infusion center and not having to come back as frequently.
It’s an agent that, at doses that are typically used, is associated with a predictable
toxicity experience. I use 1,000 mg/m2 twice daily — two weeks out of
three weeks.
![]() DR LOVE: Capecitabine generally doesn’t cause alopecia. How important is
that issue in the metastatic setting?
DR LOVE: Capecitabine generally doesn’t cause alopecia. How important is
that issue in the metastatic setting?
![]() DR CARLSON: That’s very important. If you’re going to use sequential single
agents, it’s always nice to start with an agent that doesn’t cause alopecia. If the
woman already has established alopecia, you don’t gain from the nonalopecia
properties of the new therapy. That’s often an important component of treatment
of metastatic disease.
DR CARLSON: That’s very important. If you’re going to use sequential single
agents, it’s always nice to start with an agent that doesn’t cause alopecia. If the
woman already has established alopecia, you don’t gain from the nonalopecia
properties of the new therapy. That’s often an important component of treatment
of metastatic disease.
The other reason I often will lead with capecitabine is that many of these women, because it’s the first-line therapy, have recently been diagnosed with their metastasis. They will go through all the turmoil and psychic trauma of the new diagnosis, and in that context, often it is easier to start with an agent that has acceptable toxicity, so they can become used to the chronic nature of the disease and the need for ongoing chemotherapy with an agent that has good efficacy and doesn’t affect their quality of life to a major degree.
Track 17
![]() DR LOVE: Do you agree or disagree? “Patients with ER-negative, PRnegative
and HER2-negative tumors (triple negative) should be offered
bevacizumab and chemotherapy in the first-line metastatic setting.”
DR LOVE: Do you agree or disagree? “Patients with ER-negative, PRnegative
and HER2-negative tumors (triple negative) should be offered
bevacizumab and chemotherapy in the first-line metastatic setting.”
![]() DR CARLSON: It’s reasonable to offer such a patient chemotherapy and
bevacizumab. The best evidence we have is with paclitaxel/bevacizumab.
Kathy Miller’s other ECOG study that evaluated capecitabine with or without
bevacizumab showed a slightly higher response rate using the combination
but no advantage in terms of relapse-free survival and overall survival (Miller
2005).
DR CARLSON: It’s reasonable to offer such a patient chemotherapy and
bevacizumab. The best evidence we have is with paclitaxel/bevacizumab.
Kathy Miller’s other ECOG study that evaluated capecitabine with or without
bevacizumab showed a slightly higher response rate using the combination
but no advantage in terms of relapse-free survival and overall survival (Miller
2005).
We may be seeing specific drug effects and different drug interactions between bevacizumab and chemotherapy. It may be a result of different patient populations. The patients in the capecitabine study were treated in the second-line setting, not the first-line setting, as with paclitaxel plus bevacizumab.
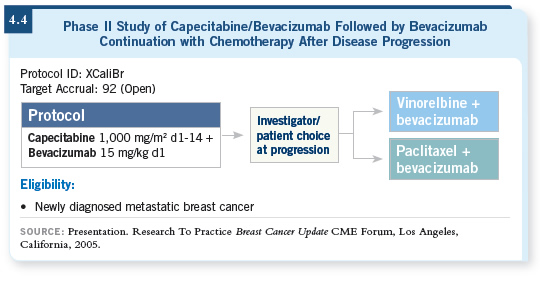
![]() DR LOVE: George Sledge is conducting a study right now of first-line
capecitabine with bevacizumab (4.4).
DR LOVE: George Sledge is conducting a study right now of first-line
capecitabine with bevacizumab (4.4).
![]() DR CARLSON: That’s an important study. Based on the existing data evaluating
capecitabine/bevacizumab, currently I’m not combining bevacizumab
and capecitabine. We didn’t see an advantage and although most patients
tolerate bevacizumab well, it does have toxicity and expense. So I’m limiting
bevacizumab use at the current time to concurrent use with paclitaxel.
DR CARLSON: That’s an important study. Based on the existing data evaluating
capecitabine/bevacizumab, currently I’m not combining bevacizumab
and capecitabine. We didn’t see an advantage and although most patients
tolerate bevacizumab well, it does have toxicity and expense. So I’m limiting
bevacizumab use at the current time to concurrent use with paclitaxel.