You are here: Home: BCU 6 | 2005: Power Point Journal Club
| |
|
|
| |

This PowerPoint Journal reviews recently published clinical research articles and presentations. In this issue, we review an article by Davide Mauri et al on a meta-analysis of neoadjuvant versus adjuvant systemic therapy and a report by Ivo Olivotto et al on a population-based validation of the Adjuvant! program for early breast cancer.
PowerPoint Journal Club slides are provided in two formats, in this monograph and on the enhanced CD. The slide presentation on the CD is designed for optimal viewing on a large screen in a dark room (below, right) and represents top-line data and information from the figures in this book. This format of PowerPoint can be difficult to read in print, and consequently, the version below has been designed to facilitate ease of reading and comprehension.

| 4.1 |
|
| |
|
 SLIDE 4.1 Trastuzumab in combination with standard chemotherapy has resulted in improvement in time to progression, overall response, duration of response and survival in patients with HER2-positive metastatic breast cancer. No randomized preoperative study has previously been performed with trastuzumab. SLIDE 4.1 Trastuzumab in combination with standard chemotherapy has resulted in improvement in time to progression, overall response, duration of response and survival in patients with HER2-positive metastatic breast cancer. No randomized preoperative study has previously been performed with trastuzumab.
|
| |
|
|
| 4.3 |
|
| |
|
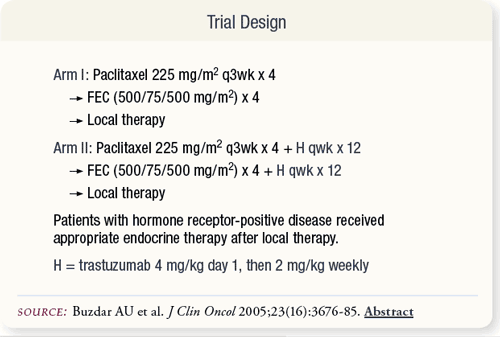 SLIDE 4.3 The dose and schedule of paclitaxel was based on information available at the study’s inception in 1999. Based on more current evidence, a weekly schedule may be more effective. Epirubicin was chosen in an attempt to reduce the cardiotoxicity attributed to trastuzumab/doxorubicin.
SLIDE 4.3 The dose and schedule of paclitaxel was based on information available at the study’s inception in 1999. Based on more current evidence, a weekly schedule may be more effective. Epirubicin was chosen in an attempt to reduce the cardiotoxicity attributed to trastuzumab/doxorubicin.
|
| |
|
|
| 4.4 |
|
| |
|
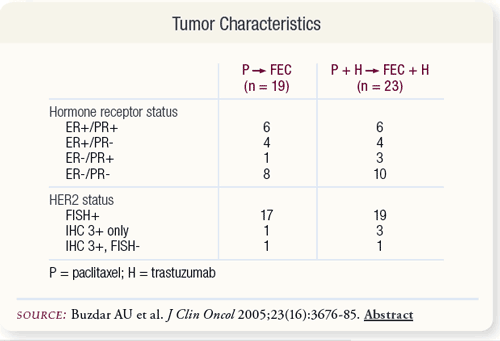 SLIDE 4.4 Approximately half of the patients had hormone receptor- positive tumors. Most patients had HER2 status of tumors confirmed by FISH. For four patients, HER2 status was determined by IHC only. One patient in each arm was subsequently found to be HER2-negative by FISH.
SLIDE 4.4 Approximately half of the patients had hormone receptor- positive tumors. Most patients had HER2 status of tumors confirmed by FISH. For four patients, HER2 status was determined by IHC only. One patient in each arm was subsequently found to be HER2-negative by FISH.
|
| |
|
|
| 4.5 |
|
| |
|
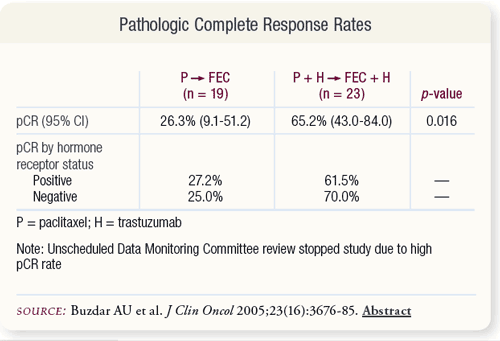 SLIDE 4.5 Among the total patients, 26.3 percent of patients in the chemotherapy alone arm achieved pCR compared to 65.2 percent of the patients treated with trastuzumab plus chemotherapy. Patients with hormone receptor-positive and -negative disease had similar pCR rates compared to the overall population.
SLIDE 4.5 Among the total patients, 26.3 percent of patients in the chemotherapy alone arm achieved pCR compared to 65.2 percent of the patients treated with trastuzumab plus chemotherapy. Patients with hormone receptor-positive and -negative disease had similar pCR rates compared to the overall population.
|
| |
|
|
| 4.6 |
|
| |
|
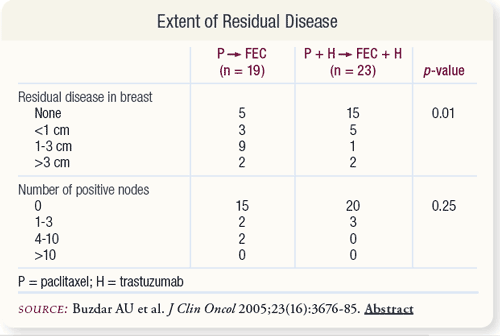 SLIDE 4.6 For patients treated with trastuzumab plus chemotherapy, the size of residual tumors in the breast was significantly smaller compared to tumors of patients treated with chemotherapy alone. The difference in the extent of residual disease in the lymph nodes was not statistically significant.
SLIDE 4.6 For patients treated with trastuzumab plus chemotherapy, the size of residual tumors in the breast was significantly smaller compared to tumors of patients treated with chemotherapy alone. The difference in the extent of residual disease in the lymph nodes was not statistically significant.
|
| |
|
|
| 4.7 |
|
| |
|
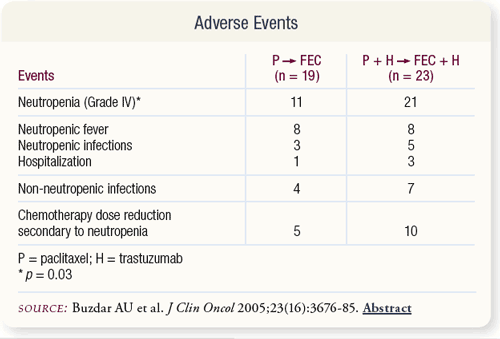 SLIDE 4.7 A higher fraction of patients treated with trastuzumab plus chemotherapy experienced Grade IV neutropenia while receiving paclitaxel. A small number of patients had neutropenic fever requiring hospitalization. There were no treatmentrelated deaths.
SLIDE 4.7 A higher fraction of patients treated with trastuzumab plus chemotherapy experienced Grade IV neutropenia while receiving paclitaxel. A small number of patients had neutropenic fever requiring hospitalization. There were no treatmentrelated deaths.
|
| |
|
|
| 4.8 |
|
| |
|
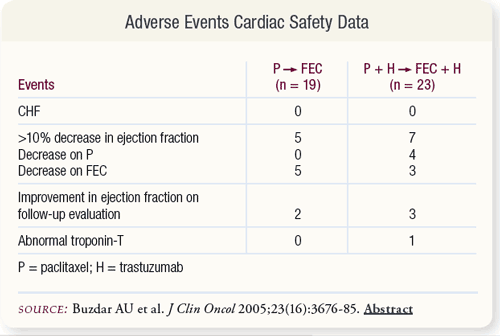 SLIDE 4.8 None of the patients developed clinical congestive heart failure. A greater than 10 percent decrease in the left ventricular ejection fraction occurred in five and seven patients in the chemotherapy alone and trastuzumab plus chemotherapy arms, respectively.
SLIDE 4.8 None of the patients developed clinical congestive heart failure. A greater than 10 percent decrease in the left ventricular ejection fraction occurred in five and seven patients in the chemotherapy alone and trastuzumab plus chemotherapy arms, respectively.
|
| |
|
|
| 4.9 |
|
| |
|
 SLIDE 4.9 These results represent the highest reported pCR rate in this patient population. The most logical explanation for this high pCR rate is the use of two potentially noncross-resistant chemotherapies in combination with trastuzumab. Another possibility is the longer duration of neoadjuvant therapy.
SLIDE 4.9 These results represent the highest reported pCR rate in this patient population. The most logical explanation for this high pCR rate is the use of two potentially noncross-resistant chemotherapies in combination with trastuzumab. Another possibility is the longer duration of neoadjuvant therapy.
|
| |
|
|
Select publications
| 5.1 |
|
| |
|
 SLIDE 5.1 This study sought to determine the safety and efficacy of capecitabine monotherapy in the treatment of advanced breast cancer in older women. SLIDE 5.1 This study sought to determine the safety and efficacy of capecitabine monotherapy in the treatment of advanced breast cancer in older women.
|
| |
|
|
| 5.3 |
|
| |
|
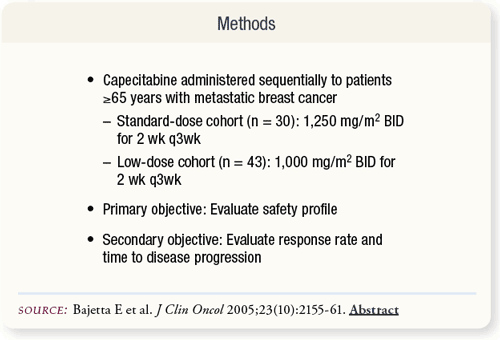 SLIDE 5.3 Patients age 65 or older with metastatic breast cancer received either standard- or low-dose capecitabine therapy. The primary objective was to assess the safety profile of capecitabine. The secondary objective was to determine efficacy in terms of response rate and time to disease progression.
SLIDE 5.3 Patients age 65 or older with metastatic breast cancer received either standard- or low-dose capecitabine therapy. The primary objective was to assess the safety profile of capecitabine. The secondary objective was to determine efficacy in terms of response rate and time to disease progression.
|
| |
|
|
| 5.4 |
|
| |
|
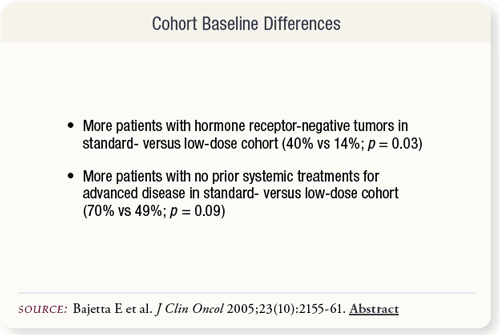 SLIDE 5.4 Baseline characteristics were similar between cohorts with the exceptions that the standard-dose cohort had a greater percentage of patients with hormone receptor-negative tumors and a greater percentage of patients not having received prior systemic treatments for advanced disease.
SLIDE 5.4 Baseline characteristics were similar between cohorts with the exceptions that the standard-dose cohort had a greater percentage of patients with hormone receptor-negative tumors and a greater percentage of patients not having received prior systemic treatments for advanced disease.
|
| |
|
|
| 5.5 |
|
| |
|
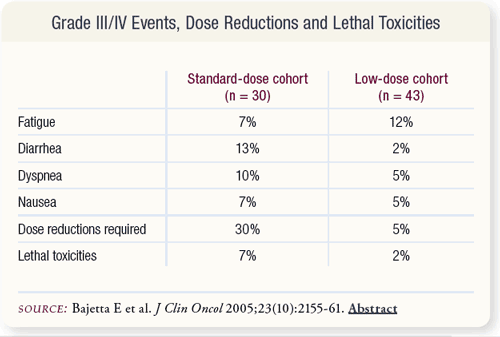 SLIDE 5.5 The initial planned dose of capecitabine for this study was 1,250 mg/m2 BID. Due to the occurrence of two toxic deaths, the dose was subsequently reduced to 1,000 mg/m2 BID.
SLIDE 5.5 The initial planned dose of capecitabine for this study was 1,250 mg/m2 BID. Due to the occurrence of two toxic deaths, the dose was subsequently reduced to 1,000 mg/m2 BID.
|
| |
|
|
| 5.6 |
|
| |
|
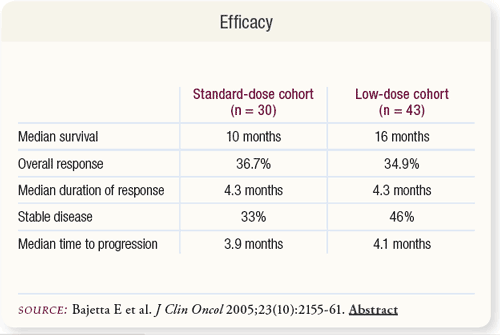 SLIDE 5.6 The response rate was 36.7 percent for the standard-dose cohort. Seven patients had disease stabilization at ≥24 weeks. The response rate was 34.9 percent in the low-dose cohort. An additional 15 patients had prolonged stabilization. Median time to disease progression was approximately four months in each group.
SLIDE 5.6 The response rate was 36.7 percent for the standard-dose cohort. Seven patients had disease stabilization at ≥24 weeks. The response rate was 34.9 percent in the low-dose cohort. An additional 15 patients had prolonged stabilization. Median time to disease progression was approximately four months in each group.
|
| |
|
|
| 5.7 |
|
| |
|
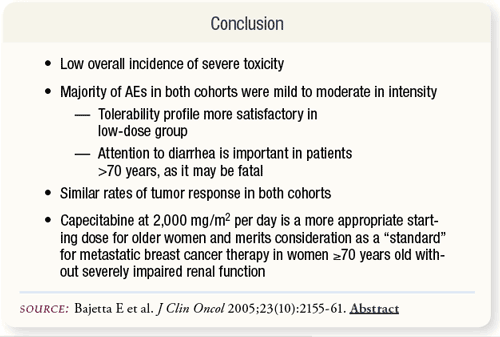 SLIDE 5.7 Due to the more favorable tolerability profile and similar rate of tumor response, low-dose capecitabine merits consideration as “standard” therapy in this patient population.
SLIDE 5.7 Due to the more favorable tolerability profile and similar rate of tumor response, low-dose capecitabine merits consideration as “standard” therapy in this patient population.
|
| |
|
|
Select publications
| 6.1 |
|
| |
|
 SLIDE 6.1 One third of new invasive breast cancer diagnoses are in women under 50 years old. This trend may increase because of changes in lifestyle, demographics and screening. In this review, Dellapasqua S et al discuss the current status of adjuvant endocrine therapy for premenopausal women with early breast cancer. SLIDE 6.1 One third of new invasive breast cancer diagnoses are in women under 50 years old. This trend may increase because of changes in lifestyle, demographics and screening. In this review, Dellapasqua S et al discuss the current status of adjuvant endocrine therapy for premenopausal women with early breast cancer.
|
| |
|
|
| 6.2 |
|
| |
|
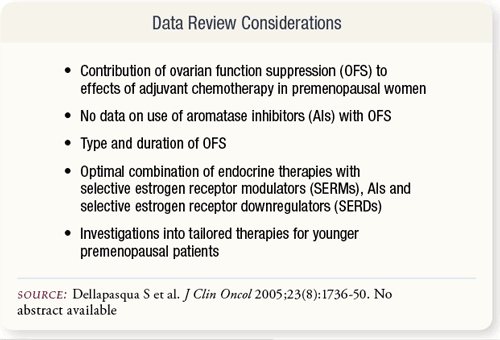 SLIDE 6.2 This review considered treatment effects of adjuvant chemotherapy, use of AIs combined with OFS, the type and duration of OFS, endocrine therapy combined with SERMs, AIs and SERDs and tailored treatments for younger versus older premenopausal women. SLIDE 6.2 This review considered treatment effects of adjuvant chemotherapy, use of AIs combined with OFS, the type and duration of OFS, endocrine therapy combined with SERMs, AIs and SERDs and tailored treatments for younger versus older premenopausal women.
|
| |
|
|
| 6.3 |
|
| |
|
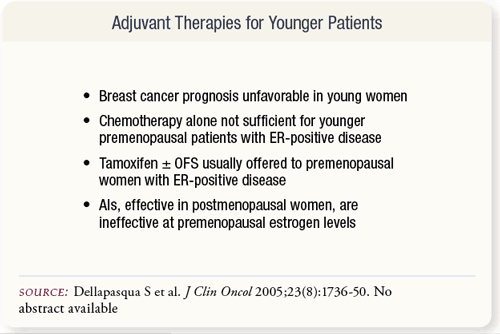 SLIDE 6.3 Adjuvant chemotherapy is usually prescribed before endocrine therapy for women with hormone-responsive disease with a high risk of relapse. Younger patients have a poorer prognosis, and AIs, effective in postmenopausal women, are ineffective in the presence of premenopausal estrogen levels. SLIDE 6.3 Adjuvant chemotherapy is usually prescribed before endocrine therapy for women with hormone-responsive disease with a high risk of relapse. Younger patients have a poorer prognosis, and AIs, effective in postmenopausal women, are ineffective in the presence of premenopausal estrogen levels.
|
| |
|
|
| 6.4 |
|
| |
|
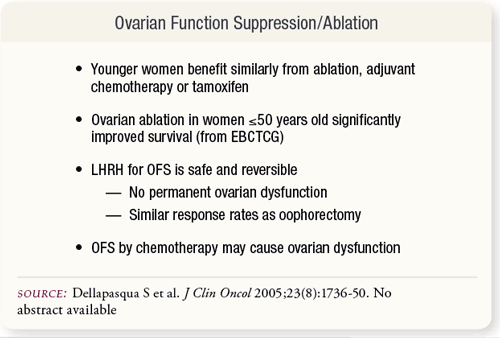 SLIDE 6.4 Adjuvant therapy of ovarian ablation with surgery or radiation improves recurrence-free and overall survival among breast cancer patients ≤50 years old. OFS is achievable with LHRH agonists and chemotherapy. Whether or not chemotherapy benefits are due entirely to endocrine effects is unknown. SLIDE 6.4 Adjuvant therapy of ovarian ablation with surgery or radiation improves recurrence-free and overall survival among breast cancer patients ≤50 years old. OFS is achievable with LHRH agonists and chemotherapy. Whether or not chemotherapy benefits are due entirely to endocrine effects is unknown.
|
| |
|
|
| 6.5 |
|
| |
|
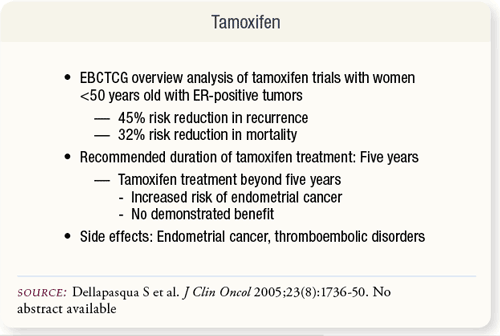 SLIDE 6.5 Results of many trials evaluating adjuvant tamoxifen support its use as adjuvant therapy for both pre- and postmenopausal women, especially those with hormone receptor-positive breast cancer. Side effects of tamoxifen include endometrial cancer and thromboembolic disorders. SLIDE 6.5 Results of many trials evaluating adjuvant tamoxifen support its use as adjuvant therapy for both pre- and postmenopausal women, especially those with hormone receptor-positive breast cancer. Side effects of tamoxifen include endometrial cancer and thromboembolic disorders.
|
| |
|
|
| 6.6 |
|
| |
|
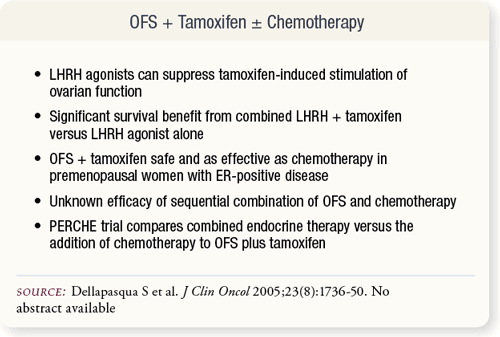 SLIDE 6.6 LHRH agonists suppress ovarian function stimulation by tamoxifen. Combined, these two agents significantly benefit survival while being safe and as effective as chemotherapy in premenopausal women with ER-positive disease. At present, the efficacy of combined OFS and chemotherapy is unknown. SLIDE 6.6 LHRH agonists suppress ovarian function stimulation by tamoxifen. Combined, these two agents significantly benefit survival while being safe and as effective as chemotherapy in premenopausal women with ER-positive disease. At present, the efficacy of combined OFS and chemotherapy is unknown.
|
| |
|
|
| 6.7 |
|
| |
|
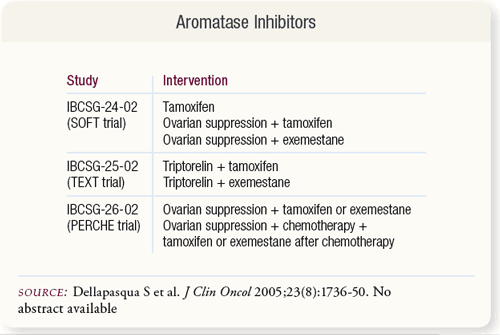 SLIDE 6.7 Aromatase inhibitors in combination with GnRH analogs may be effective as adjuvant therapy for premenopausal women with endocrine-responsive breast cancer. This is being investigated in the ongoing SOFT, TEXT and PERCHE trials coordinated by the International Breast Cancer Study Group (IBCSG). SLIDE 6.7 Aromatase inhibitors in combination with GnRH analogs may be effective as adjuvant therapy for premenopausal women with endocrine-responsive breast cancer. This is being investigated in the ongoing SOFT, TEXT and PERCHE trials coordinated by the International Breast Cancer Study Group (IBCSG).
|
| |
|
|
| 6.8 |
|
| |
|
 SLIDE 6.8 There are special issues to consider: when provided with the option, premenopausal women would prefer a GnRH analog over chemotherapy; preservation of ovarian function during chemotherapy, including ovarian tissue preservation; and safety of endocrine therapies in specific subgroups of women. SLIDE 6.8 There are special issues to consider: when provided with the option, premenopausal women would prefer a GnRH analog over chemotherapy; preservation of ovarian function during chemotherapy, including ovarian tissue preservation; and safety of endocrine therapies in specific subgroups of women.
|
| |
|
|
| 6.9 |
|
| |
|
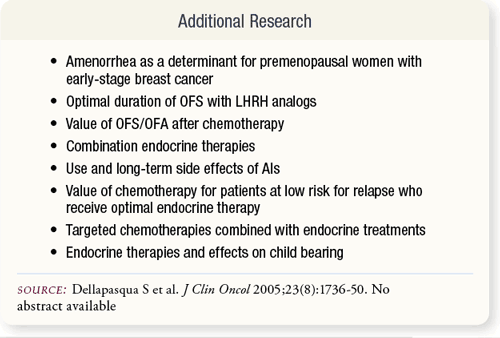 SLIDE 6.9 There are special issues to consider: when provided with the option, premenopausal women would prefer a GnRH analog over chemotherapy; preservation of ovarian function during chemotherapy, including ovarian tissue preservation; and safety of endocrine therapies in specific subgroups of women. SLIDE 6.9 There are special issues to consider: when provided with the option, premenopausal women would prefer a GnRH analog over chemotherapy; preservation of ovarian function during chemotherapy, including ovarian tissue preservation; and safety of endocrine therapies in specific subgroups of women.
|
| |
|
|
Select publications
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

