Jack Cuzick, PhD |
EDITED COMMENTS |
 ATAC trial: 68-month results ATAC trial: 68-month results
Efficacy
The most important results were that the effects were maintained up to and beyond the five years of active treatment. Evidence exists of a “carryover” effect with tamoxifen, which isn’t surprising. The initial results from the ATAC trial suggest that the effect will be even larger for anastrozole. The fact that the six-year absolute difference in recurrence rates between anastrozole and tamoxifen is larger than the five-year difference and the curves are still separating is particularly exciting.
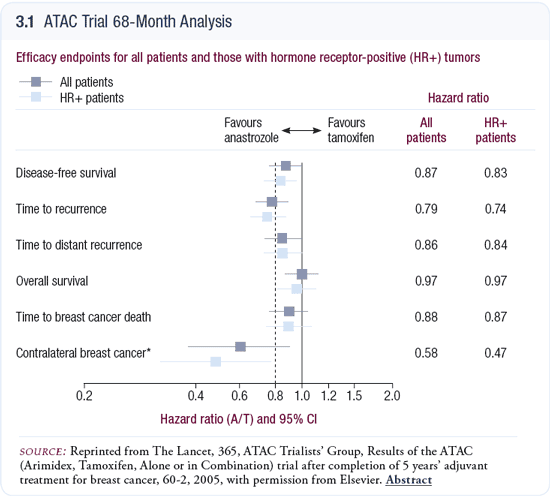
At 68 months of follow-up, no difference in overall mortality and a 12 percent nonsignificant (p = 0.2) reduction in breast cancer deaths were noted with adjuvant anastrozole compared to tamoxifen (Howell 2005; [3.1]). It is early to expect a difference in survival. The significant improvement in distant disease-free survival with anastrozole will likely translate into a reduction in breast cancer mortality in a few more years.
Of course, the mortality benefit will be attenuated because — just as with tamoxifen — the mortality benefit is about half the recurrence benefit, as women take treatment upon recurrence if they haven’t received it as adjuvant therapy. The same will be true in this situation; women who didn’t receive an adjuvant aromatase inhibitor will receive it upon recurrence. You’d expect the mortality benefit to be about half of the recurrence benefit. A 10 to 15 percent reduction in relative mortality is what one might anticipate.
Safety
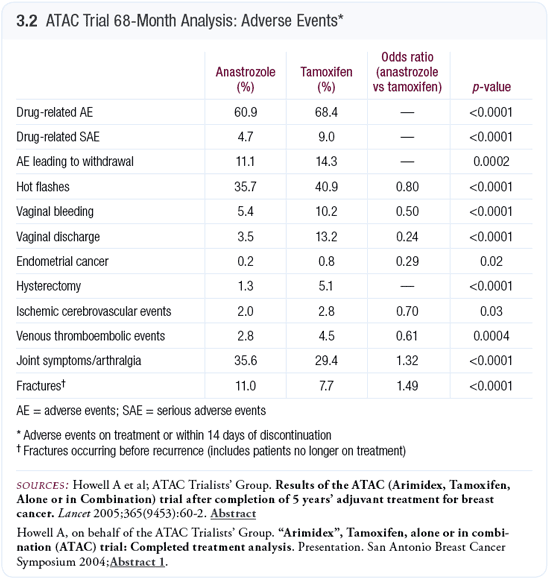
In the IBIS-1 trial, we found an increase in the hysterectomy rate for patients treated with tamoxifen (Cuzick 2002), thus it wasn’t surprising to find the same in the ATAC trial. The actual magnitude was surprising, however. The hysterectomy rate was about four times as high with tamoxifen in the ATAC trial (Howell 2005; [3.2]), whereas we saw roughly a doubling in the prevention trial (Cuzick 2002). We’re looking at the hysterectomy rates in different countries. One might suspect it’s going to be high in the United States, where more endometrial monitoring occurs.
Potential effect of tamoxifen on the progesterone receptor
My belief is that when patients with ER- and PR-positive disease receive adjuvant tamoxifen, the first negative event for them is the loss of the progesterone receptor. Of course, we don’t actually see that, because it occurs in the micrometastases. The loss of the progesterone receptor occurs progressively at a high rate over the first two to three years of tamoxifen use, and once it happens, the rate of metastases is about double what it would be in patients who have both receptors.
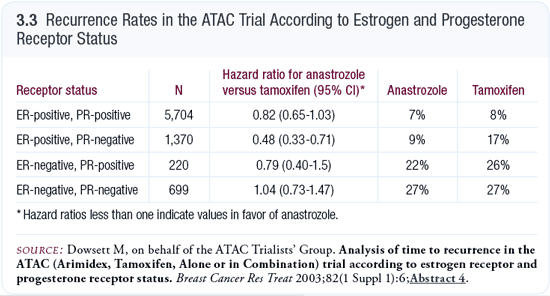
In the patients with ER-positive and PR-negative disease in the ATAC trial, the recurrence rate was about half for those treated with anastrozole compared to those treated with tamoxifen (Dowsett 2003; [3.3]). For patients with ER-positive and PR-negative disease, tamoxifen doesn’t work well, but anastrozole works as well as in the patients with ER- and PR-positive disease. This is just a model, however; we need more data to flesh this out.
If the model is correct, it suggests that starting with an aromatase inhibitor is best for all patients because you don’t have the priming effect that tamoxifen causes. Tamoxifen is pushing some patients into a poorer prognosis group. You’re always better off using the best treatment first. That will be particularly apparent if the patient has PR-negative disease initially. If the patient has PR-positive disease initially, it may take longer for that effect to be demonstrated, but I believe that it’s never better to use tamoxifen first.
Recent adjuvant aromatase inhibitor trials
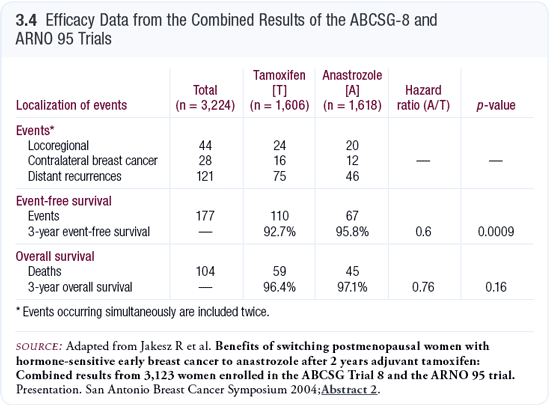
The German/Austrian studies (ARNO 95/ABCSG-8) evaluated the use of anastrozole after two years of adjuvant tamoxifen (Jakesz 2004; [3.4]). The results were almost exactly in line with the exemestane study (Coombes 2004), suggesting that after two to three years of adjuvant tamoxifen, exemestane and anastrozole have essentially equivalent efficacy. In the BIG 1-98 trial comparing initial adjuvant therapy with letrozole to tamoxifen (Thürlimann 2005a, 2005b), the efficacy results in patients with ER-positive disease were almost identical to the results from the ATAC trial (Howell 2005).
BIG 1-98 trial
Originally, the BIG 1-98 trial, which accrued about 1,800 patients, was going to compare five years of adjuvant therapy with letrozole or tamoxifen. However, at a later stage, the IBCSG decided to evaluate the crossover. In the remaining 6,000 patients, the trial was essentially a two-by-two design. Patients began adjuvant therapy with either tamoxifen or letrozole and after two years, they were randomly re-assigned to continue with their initial treatment or switch to the other treatment (Thürlimann 2005a, 2005b; [3.5]).
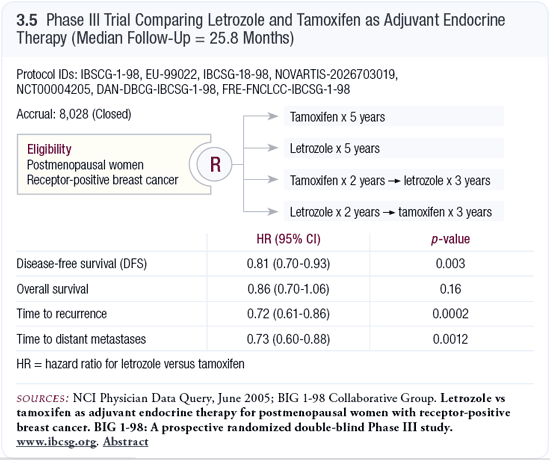
We don’t know anything about the switch. Strong evidence is emerging that at some stage, an aromatase inhibitor is a good idea; the only arm in question is the one in which patients start and remain on tamoxifen. In my view, it would be difficult to justify continuing with five years of tamoxifen; however, the other three arms are important and will provide the first evidence about switching from an aromatase inhibitor to tamoxifen.
The initial results from BIG 1-98 have been reported — a comparison between tamoxifen and letrozole in which all patients who were switched are censored. The efficacy results were essentially the same as those in the ATAC trial at the 30-month point. The hazard reduction was similar, and the side-effect profile was by and large the same, although it was reported differently (Thürlimann 2005a, 2005b).
A few differences were seen. They found a benefit for letrozole only in patients with node-positive disease, which is difficult to understand. It’s probably a chance finding, but we need to follow that. At this stage, they’ve found no difference in efficacy between the patients with PR-positive and PR-negative disease (Thürlimann 2005a, 2005b). We have to acknowledge that the data are different from what’s been observed in other trials.
The third and most worrying finding is the substantial excess in cardiovascular deaths for letrozole compared to tamoxifen (Thürlimann 2005a, 2005b), which hasn’t been observed in the trials with anastrozole. Whether this is due to chance or differences in cardiovascular mortality is important to know. Letrozole is a slightly more potent aromatase inhibitor, and it is not clear whether that has an impact.
ATAC trial: Cardiovascular mortality
In the IBIS prevention trials, I’m glad we are using anastrozole because there’s no worry about cardiovascular safety. If one were to consider letrozole for prevention now, I would be concerned about proceeding until I could see how the data panned out.
We have cardiovascular mortality data from the 68-month follow-up of the ATAC trial that have not yet been presented; they are in a paper about to be submitted. In fact, the data were in the paper of the 68-month follow-up we initially offered for publication, but The Lancet and the New England Journal of Medicine wanted a shorter publication, and we only presented the headline results.
Incidence of contralateral breast cancer in trials of adjuvant aromatase inhibitors
The study results have been mixed in terms of the aromatase inhibitors’ effects on overall recurrence, primarily because of the different designs. The studies that have sequenced aromatase inhibitors after tamoxifen have shown bigger relative effects, but that may be explained by the effects on the receptors. Overall, when you consider the incidence of contralateral tumors, the trials are remarkably similar. The trials are showing nearly a 50 percent reduction in the incidence of contralateral tumors for patients treated with an aromatase inhibitor compared to those treated with tamoxifen (Howell 2005; Coombes 2004). However, in the MA17 trial, letrozole was compared to a tamoxifen carryover effect (Goss 2003).
This bodes well for prevention. First of all, I’m excited that the effect on the incidence of contralateral tumors is consistently larger than the effects on the incidence of recurrence (50 percent versus 25 to 40 percent). Aromatase inhibitors are expected to only have an effect on new tumors that are ER-positive. We don’t have data on the receptor status of the contralateral tumors, although the data will be available soon from the ATAC trial. Overall, a 50 percent reduction above and beyond tamoxifen’s ability to reduce the incidence of ER-positive tumors by 50 percent would suggest a 75 percent reduction in new ER-positive tumors. Eradicating 75 percent of ER-positive breast cancer would be fantastic.
Optimal duration of therapy with adjuvant aromatase inhibitors
We know virtually nothing about the optimal duration of adjuvant therapy with the aromatase inhibitors. That will be the major question in the next round of trials. Many of the trials have reported large effects after a couple years of aromatase inhibitors. The ATAC trial has gone out to five years. There’s no reason to stop at five years. The side-effect profile looks good. If they are used longer, a DEXA scan will be needed to keep an eye on the bones. The issue of longer duration of therapy is both important and useful.
No direct evidence suggests that five years is better than two years. Many believe that 10 years is going to be better than five. The MA17 trial is the one example in which the duration of therapy is being evaluated. All of those patients had five years of tamoxifen, and the patients who were treated with letrozole after five years of tamoxifen will be randomly re-assigned at 10 years to stop or continue letrozole. Hence, it will be a trial of five years of tamoxifen followed by either five or 10 years of letrozole.
Managing bone loss associated with the aromatase inhibitors
Great strides have been made in terms of the new bisphosphonates. The oral weekly preparations are well tolerated. I am optimistic that bone loss is completely manageable, and it may actually lead to a greater public health benefit by paving the way for having osteoporosis dealt with routinely in all postmenopausal women. That could be one of the more beneficial effects of this issue. With the new bisphosphonates and the potential availability of DEXA scans, osteoporosis may be a disease of the past in another decade.
Select publications
 |
Dr Cuzick is the John Snow Professor of Epidemiology at the Cancer Research UK Centre for Epidemiology, Mathematics and Statistics, Wolfson Institute of Preventive Medicine, Barts and the London, Queen Mary’s School of Medicine and Dentistry in London, United Kingdom. |
|

