Norman Wolmark, MD |
EDITED COMMENTS |
 The Oncotype DX multigene assay The Oncotype DX multigene assay
Development of Oncotype DX
We were intrigued by the possibility of developing a predictive assay using formalin-fixed, paraffin-embedded tissue, because we had an extensive annotated tissue library collected from women who had been treated in a relatively uniform manner, for whom we had decades of follow-up.
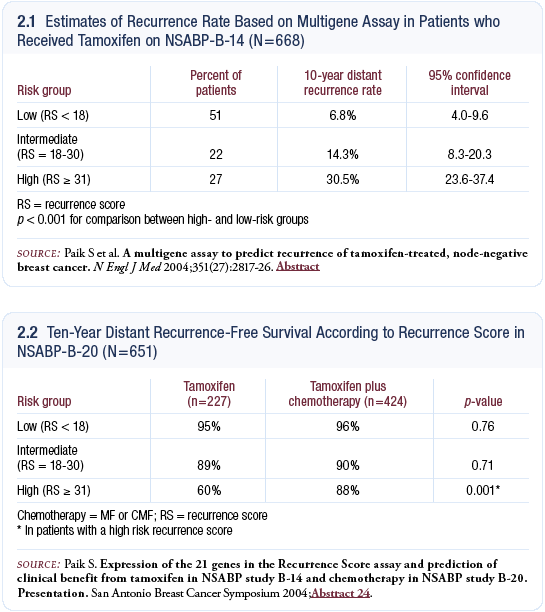
Genomic Health Inc selected 250 candidate genes from microarray data and genomics databases and then assessed them in three separate studies with nearly 500 patients. From univariate analyses, they defined 16 genes that were closely associated with recurrence and then, using various statistical techniques, grouped them into four separate categories, applied weights and developed a recurrence-score algorithm.
Scores less than 18 were low risk, 18 to 30 were intermediate and greater than 31 were high risk. The assay was then validated prospectively in NSABP-B-14, which randomly assigned patients with node-negative, receptor-positive disease to tamoxifen versus placebo. In patients on the tamoxifen arm, robust differences were seen between the patients with low-risk and high-risk disease. The 10-year distant recurrence-free survival was 93 percent in the low risk group and 69 percent in the high-risk group (Paik 2004a; [2.1]). The assay is now commercially available as Oncotype DX.
Benefit of chemotherapy based on recurrence score
We wanted to determine whether the assay could predict the benefit of chemotherapy, so we examined the data from NSABP-B-20, which randomly assigned patients with receptor-positive, node-negative disease to tamoxifen versus tamoxifen plus CMF chemotherapy versus tamoxifen plus MF chemotherapy. We found that patients at high risk derived benefit from chemotherapy, but patients at low risk, who comprised 50 percent of the cohort, did not appear to derive substantial benefit from the addition of chemotherapy to tamoxifen (2.2).
The intermediate group comprised only 20 to 25 percent of the cohort, and we didn’t have the power to determine if they benefit from the addition of chemotherapy. We were surprised to find that the relative risk reduction was not uniform — different risk groups did not have the same relative risk reduction. The greatest relative risk reduction was seen in patients at high risk.
Quantitative estrogen receptor expression and benefit of tamoxifen
It appears we can associate the benefit of tamoxifen with specific objective molecular discriminants. Quantitative ER expression, as determined by RT-PCR, correlates with benefit — the greater the quantitative ER, the greater the likelihood of benefit from tamoxifen. We’re using quartiles at this point, and patients who fall into the highest quartile, as determined by RT-PCR, receive the greatest benefit from tamoxifen. That doesn’t justify not treating ER-positive patients with tamoxifen; however, the expected benefit is relative. We will explore the possibility of utilizing RT-PCR, rather than ER/PR status as determined by IHC, when assessing whether to use hormonal therapy.
NSABP-B-32 sentinel node study
The preliminary specificity and sensitivity data from NSABP-B-32 with over 5,500 women demonstrated a nine to 10 percent false-negative rate for detecting positive nodes with the sentinel node resection (Julian 2004). One can say that surgeons with more experience have a lower rate or that if we examine two or three sentinel nodes, we can lower that rate; however, examining four to five nodes is comparable to performing an axillary node dissection. If you absolutely need to know whether the nodes are positive or negative, will you accept a nine to 10 percent likelihood of a false negative?
NSABP-B-39: Proposed Phase III trial of conventional whole breast irradiation versus partial breast irradiation
NSABP-B-39 will evaluate whole breast irradiation compared to partial breast irradiation (PBI) in a sample of approximately 3,000 patients. Being able to replace a six-week course of radiotherapy for whole breast irradiation with shorter-term PBI, without increasing the likelihood of ipsilateral breast tumor recurrence, would be beneficial.
MammoSite provides a potentially attractive method of administering interstitial PBI because one can envision percutaneous ablation of small lesions with concurrent PBI and identification of molecular markers predicting axillary involvement occurring in a single outpatient visit.
Select publications
 |
Dr Wolmark is Chairman of the Department of Human Oncology at Allegheny General Hospital, Professor and Chairman at Drexel University College of Medicine and Chairman of the National Surgical Adjuvant Breast and Bowel Project in Pittsburgh, Pennsylvania. |
|

