Gabriel N Hortobagyi, MD |
EDITED COMMENTS |
 Aromatase inhibitors as initial adjuvant therapy in postmenopausal women Aromatase inhibitors as initial adjuvant therapy in postmenopausal women
The third generation aromatase inhibitors are better than tamoxifen, and my postmenopausal patients with ER-positive disease who have not yet started adjuvant hormonal therapy will initially receive an adjuvant aromatase inhibitor — preferably anastrozole. We started using adjuvant anastrozole instead of tamoxifen after the first presentation of the ATAC trial results.
Even if tamoxifen and anastrozole had been therapeutically equivalent, anastrozole would still be preferable because it was better tolerated. For us, the issue of osteopenia was always secondary. We already had experience with the bisphosphonates and monitoring patients for osteoporosis, because chemotherapy and ovarian ablation produce premature menopause and accelerated bone resorption. We felt quite comfortable in switching our front-line adjuvant therapy to anastrozole.
Aromatase inhibitors as crossover therapy in postmenopausal women receiving adjuvant tamoxifen
Our current practice in postmenopausal women who are taking adjuvant tamoxifen for any length of time is to switch to an aromatase inhibitor. We try to conform to the data from the randomized trials evaluating a crossover to the aromatase inhibitors, but taken together, those data appear to have a class effect.
A year ago, I would have said, “If the woman had received adjuvant tamoxifen for five years, I would switch to letrozole. If the woman had received adjuvant tamoxifen for two or three years, I would switch to exemestane.” Right now, I feel comfortable with any of the aromatase inhibitors at any point in time of switching. In addition to the MA17 trial with letrozole (Goss 2003) and the Intergroup Exemestane Study (Coombes 2004), the Italian trial (Boccardo 2003) with anastrozole reported similar results*. In the absence of a head-to-head comparison, the toxicity profiles of these three drugs are very similar.
Aromatase inhibitors following five years of adjuvant tamoxifen
We base this decision on the patient’s risk. In some patients the residual risk is so small that the benefit of additional therapy is marginal. Yet some of these patients have difficulty letting go of tamoxifen — it’s a safety net and they want to continue on adjuvant therapy. Others can’t wait to finish the treatment.
At ASCO 2004, I presented an abstract on the prognosis of patients with operable breast cancer five years after diagnosis (Hortobagyi 2004; [3.1]). We pooled our adjuvant data dating back to 1974 from approximately 2,500 patients who had received adjuvant therapy and replotted the survivors’ disease-free survival five years after diagnosis.
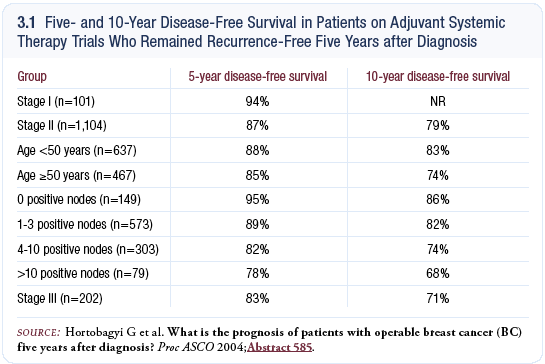
We studied the pattern of relapse in the second five years and found that for most patients with Stage II and Stage III disease, the residual risk is sufficient to justify additional therapy, including patients with ER-positive tumors who received five years of tamoxifen.
If I believe a patient has a sufficiently high risk, I will consider offering an aromatase inhibitor six or even 18 months after she completed five years of tamoxifen. Where to draw the line is gray, because we don’t have good data on how to calculate residual risk at the end of five years of tamoxifen. We are rather proficient at calculating risk at the time of initial diagnosis by using Chuck Loprinzi’s model or Peter Ravdin’s Adjuvant! program, but we’re not very good at determining risk five years later.
Perspective on sentinel lymph node biopsy
It is apparent to me that despite not having completed the controlled trials, sentinel lymph node biopsy has become an accepted standard for patients with clinically negative axillary lymph nodes. Use of this procedure has progressed much faster than the actual clinical trials. I’m comfortable with the idea that you can safely spare a number of patients more extensive axillary surgery — clearly the most morbid part of breast surgery — if patients have clinically negative lymph nodes, especially if imaging studies don’t reveal anything in the axilla and the sentinel lymph node biopsy is negative.
I’m less comfortable with what to do with the information we are obtaining from these procedures. We are nowhere close to understanding the meaning of microscopic deposits or loose cells in an extensively tested sentinel lymph node. I hear more questions and see more variability about what to do with that information than virtually any other aspect of locoregional therapy.
This worries me because I suspect many patients are being overtreated on the basis of these findings, because the practitioner — with the best of intentions — wants to give the patient the benefit of the doubt. As soon as the pathologist reports one suspicious cell in that sentinel lymph node, it is difficult to backpedal. It is critical for us to complete the NSABP and the American College of Surgeons trials, which evaluate sentinel lymph node biopsy and the implications of the diagnostic findings in terms of outcome and prognosis. This will enable us to have a unified approach to these patients.

Select publications
 |
Dr Hortobagyi is Professor of Medicine, Nellie B Connally Chair in Breast Cancer and Chairman of the Department of Breast Medical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. |
|

