Michael Baum, MD, ChM |
EDITED COMMENTS |
 ATAC trial update ATAC trial update
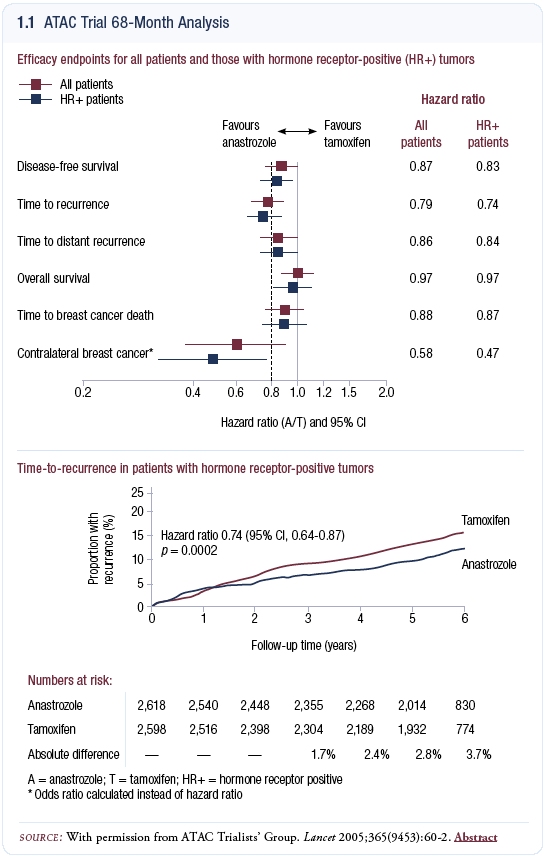
The ATAC trial has reached an important point in its evolution, with a median followup of 68 months (ATAC Trialists’ 2005; [1.1]). Almost all of the patients are now off therapy, and we have one year of follow-up after the therapy was completed. This is important for two reasons: it makes me comfortable about the efficacy and the hypothetical “carryover effect” we’ve been hearing about for tamoxifen, and it makes me comfortable with the toxicities and tolerability of anastrozole. I believe this is probably the most important of the three ATAC analyses, and it allows me, as a practicing clinician, to change practice. I speak not only as a practicing clinician but also as the past principal investigator of the trial.
The simplest interpretation of the results is that anastrozole prevents one in four of the relapses we see in patients on tamoxifen. That translates into highly significant improvements in disease-free survival, recurrence-free survival and distant disease-free survival. The absolute number for difference in recurrence-free survival in the patients with receptor-positive disease at six years is close to four percent. It is important to remember that this trial included a group of patients with a relatively good prognosis.
In terms of relative risk reductions, we have no reason to suppose that the relative risk reductions will be different in any subgroup, and if that one in four relative risk reduction is across the board, then in a subgroup of postmenopausal patients on tamoxifen with, for example, a 40 percent chance of relapse on tamoxifen at six years, the absolute reduction with anastrozole is about 10 percent, not four percent, as was seen in the ATAC trial.
ATAC overall survival data
This analysis was triggered by the number of distant recurrences and deaths from all causes. With regard to distant recurrences, our power calculations were correct; the trial was sufficiently powered to detect a significant difference. For overall survival, our power calculations were wrong, because this was a group of elderly women with a good prognosis, and the overall survival analysis is diluted by deaths from other causes before breast cancer recurrence.
I believe we can predict breast cancer survival with a fair degree of precision. I can’t see any reason why we would not eventually see a significant difference in breast cancer deaths. This trend is already present and is close to significance.
Whether that will translate into overall survival is uncertain. I’m not concerned about toxic side effects contributing to other causes of death — we know enough about anastrozole not to be worried about that — but I am concerned that the effect of preventing breast cancer deaths might be diluted by competing morbidities.
I’ve always been a purist, arguing that the only two real outcome measures in medicine are length of life and quality of life. I am on record as saying that all other outcome measures are surrogates, but we have to avoid waiting too long for the length-of-life outcome and to accept that surrogate measures translate into length of life with a fair degree of precision.
Quality of life and toxicity data in the ATAC trial
The use of anastrozole instead of tamoxifen does not impair quality of life. We can also say, with confidence, that the gynecological symptoms linked to tamoxifen have now translated into a fourfold increase in hysterectomy rates compared with anastrozole.
That is a dramatic observation, which we nearly missed. We tracked down all the hysterectomies in women who had their wombs at the time of randomization. We came up with an extraordinary figure — I believe it’s the most extreme relative risk I’ve encountered in clinical trials.
The absolute numbers were 1.3 percent versus 5.1 percent (Howell 2004) for anastrozole and tamoxifen, respectively. This has a profound economic impact. I also don’t know how many hysteroscopies are being performed for every hysterectomy or how much the workup costs to decide whether a woman should have a hysterectomy, but these are big cost issues. The update doesn’t give us any new information with regard to other prespecified adverse events, and no other adverse event is emerging with a frequency of more than one percent.
The fracture rate incidence is becoming a little more reassuring. An excess fracture rate occurs in the first two or three years, but then the lines are beginning to come together (Howell 2004). As patients stop taking anastrozole, the fracture rate returns to that of the patients randomized to tamoxifen. Furthermore, so far no difference has occurred in fractures of the neck or femur, which are of particular concern.
I think the issue of bone is easy to manage. We should be alert to it, monitor bone mineral density, perhaps exclude patients who have established osteoporosis, and then be ready to intervene with a bisphosphonate when the patient becomes osteopenic. The polyarthralgia with aromatase inhibitors remains a problem. We don’t understand it, and it occasionally leads to withdrawal of treatment; however, the bottom line is that a significant difference exists favoring anastrozole for patients withdrawing from treatment because of side effects. If you evaluate the totality of side effects, anastrozole does better. If you consider the issue of the gynecological symptoms leading to hysterectomy, I believe the new drug — anastrozole — has the better tolerability profile.
Partial breast irradiation
Three-dimensional conformal, multi-collimator radiotherapy is absurd because no matter how carefully they plan their fields, they’re not hitting the target. A number of studies using MRI have demonstrated this. Even if the surgeon puts clips around the cavity at the time of operation, the cavity collapses. It’s a slit rather than a lump, and the clips migrate. I don’t believe it will work out. I’m interested in conforming the tissue to the source, rather than the other way around. Both our TARGIT approach and the ELIOT system developed by Umberto Veronesi allow that. In theory, MammoSite® also allows the tissue to be conformed to the source, but I’ve heard that in many cases, the balloon is too close to the skin, and that leads to skin necrosis because it’s inserted through a trocar.
With Targit, a miniature electron generator accelerates electrons down a tube, hits a target and produces a sphere of soft x-rays. The longer you leave it on, the greater the field treated. After removing the tumor, an applicator sphere is selected that matches the removed tumor volume. The breast tissue is wrapped around that sphere and, by luck rather than design, it adheres by surface tension to the plastic. The electron generator tube is inserted down the center with the tip pointers in the epicenter of the sphere. The skin is everted from the shaft of the applicator and the machine is turned on. The duration of treatment varies between 20 to 30 minutes, depending on the diameter of the sphere. It can be performed in any type of operating theater. We have a tungsten-impregnated sheet around the applicator, and there’s almost no radiation beyond it. Typically, we leave the anesthetist in theater, go for a cup of coffee, then return and close up.
I am the principal investigator of TARGIT, which is a multinational trial where carefully selected patients are randomly assigned to intraoperative radiotherapy or conventional external beam radiation therapy. We’ve already enrolled 200 patients out of our target accrual of approximately 2,000.
Select publications
 |
Dr Baum is an Emeritus Professor of Surgery and Visiting Professor of Medical Humanities at University College London in the United Kingdom. |
|

