| You are here: Home: BCU 7|2004: Frank A Vicini, MD, FACR
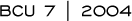
Frank A Vicini, MD, FACR |
EDITED COMMENTS |
 Development of partial breast irradiation (PBI) Development of partial breast irradiation (PBI)
In the early 1990s, patients who did not have easy access to radiation facilities did not have the option of breast conservation, so institutions began offering interstitial implants to shorten the treatment time from six and a half weeks to four days. While hundreds of these cases were performed with very good results, the procedure required four days of hospitalization, which was impractical.
High-dose rate brachytherapy was then developed — a similar interstitial procedure performed on an outpatient basis. With brachytherapy, a series of hollow catheters placed during lumpectomy or shortly thereafter encircle the surgical cavity. At the time of treatment, cables connected to a high-dose rate unit are attached to the catheters, and a computer transmits the source to a predetermined position along the length of the catheters, delivering a targeted dose of radiation around the cavity.
The whole process of transferring the source into the catheters and back into the housed radioactive unit takes five to 10 minutes. Then the cables are detached from the catheters and the patient returns six hours later for a second treatment. That procedure is repeated eight to 10 times over a course of four or five days and then the catheters are removed.
While patients tolerated this new type of interstitial therapy very well, it was unpleasant to have 15 to 20 needles placed in the breast and inconvenient to come twice daily for treatments. Approximately five or six years ago the MammoSite® was designed to deliver the same type of radiation to the lumpectomy cavity either shortly after surgery or a few weeks later. It’s an easier procedure for physicians to learn and perform, and it’s much easier on the patient, requiring only one catheter.
Alongside the development of the MammoSite®, 3-D conformal external beam radiation came into existence. When this technology is applied to breast cancer, the same areas around the lumpectomy cavity are treated with 10 fractions of radiation therapy over five days on an outpatient basis. The procedure is not invasive and we can use the same equipment that we already have in the radiation facility. At our institution, we are able to offer patients all three types of PBI and select the technique most appropriate for the individual case.
Interstitial brachytherapy
At William Beaumont Hospital, we have the largest single experience with interstitial brachytherapy (3.1). We matched 199 patients treated with interstitial brachytherapy with 199 patients who received conventional external beam radiotherapy. With a median follow-up for surviving patients of 65 months, we found the endpoints to be equivalent, including local control rates, regional failure rates and cause-specific survival.
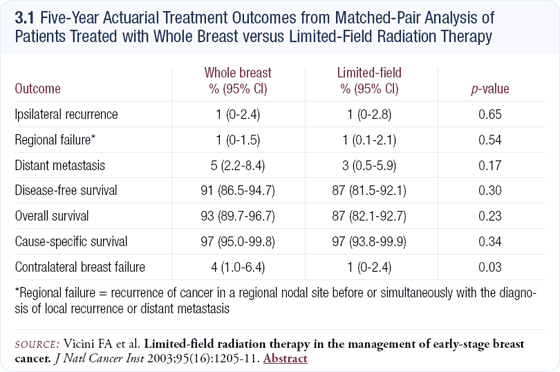
In the past 10 years of published data, the collective experience for patients treated with interstitial brachytherapy with over five years of follow-up consists of approximately 500 to 600, compared to tens of thousands of women treated with whole-breast radiation. With brachytherapy, we have only small numbers of highly selected patients treated at single institutions (3.2). We don’t really know what the efficacy will be in larger patient populations with less restrictive criteria.
Experience with PBI
One of the advantages of PBI is that it can be completed quickly before systemic therapy is begun. Our surgeons have been very progressive in this field and we’re one of the few institutions that offers interstitial brachytherapy, MammoSite® and 3-D conformal external beam radiation. Each technique has its advantages, and none of them is applicable in all clinical scenarios. Treatment must be individualized based on factors such as the patient’s access to a radiation facility and the location of the lesion within the breast.
At our institution, of the patients who receive PBI, approximately 60 percent are treated with the MammoSite®, 30 percent with conformal external beam radiotherapy and a small percentage with interstitial brachytherapy. Some have questioned whether it’s worthwhile to study PBI given the high efficacy and low toxicity achieved with breast conservation using whole breast radiation.
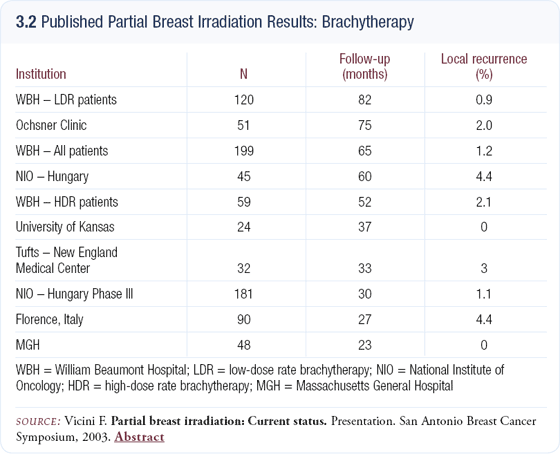
However, in the United States, a large proportion of women do not undergo breast-conserving therapy, and a recent study showed that the distance to a radiation facility still factors into a woman’s decision-making (3.3). In addition, some people fear radiation and reducing the time and, potentially, the toxicity of radiation may increase the rate of breast conservation. I believe that an additional 10 to 20 percent of women making this decision would select breast-conserving therapy if PBI was an option.
PBI for DCIS
The American Brachytherapy Society (ABS) has developed recommendations for the off-protocol use of brachytherapy. Based on the data currently available, the ideal patients are those with tumors of less than three centimeters, negative lymph nodes, negative margins and no extensive intraductal component. They exclude patients with DCIS because only a small number of such patients in single institutions have been treated with this technique, but I suspect that will change in the next few years.
The NSABP and RTOG plan to jointly conduct a study that will randomly assign 3,000 patients to conventional whole breast radiation or one of three PBI techniques — interstitial brachytherapy, MammoSite® or 3-D conformal external beam radiation. The eligibility will be broad with no age restrictions and it will include patients with DCIS. Considering the applications for PBI, I believe patients with DCIS are ideal for testing this concept because the issue of a survival disadvantage is no longer arguable. The only difference between whole breast irradiation and PBI is that with limited-field radiation, we’re targeting the tissues that most likely require it. I consider PBI a reasonable compromise between no radiation and six and a half weeks of radiation, which is probably overkill in the majority of DCIS cases.
MammoSite® procedure
The MammoSite® can be placed either during or after surgery. Approximately 50 percent of the over 1,000 patients on the MammoSite® registry had their device placed intraoperatively; however, it appears the more experienced institutions choose to place it postoperatively. I prefer the postoperative, closed-cavity technique because it allows me to ascertain whether a patient is truly a candidate for PBI — both pathologically and technically — before I discuss it with the patient. It’s distressing for someone to learn she is not eligible for PBI after a device or catheters have been placed.
Pathological reasons why a patient may be ineligible include positive nodes, large tumors, lobular histology, DCIS and positive margins, although if the margins are positive, one can re-excise and place the device at re-excision or wait for the subsequent pathology report. Technical suitability can be determined by CT, as it is important in placing the MammoSite® to keep the balloon at least five to seven millimeters away from the skin surface to avoid excessive radiation to the skin. The cavity shrinks postoperatively, so waiting one to two weeks after surgery allows us to work with a smaller cavity, which is better.
If the MammoSite® is deemed appropriate, then the procedure is performed a day or two later on an outpatient basis. The device is placed in the morning and later that day, conformance is assessed by CT. The breast tissue needs to conform well around the balloon because the dose is prescribed one centimeter away from the surface of the balloon.
Any intervening fluid or air would prevent that one-centimeter rim of tissue from receiving 100 percent of the dose. Typically treatment is then begun within 24 hours, although if the conformance is unacceptable, we can wait a day or two before beginning therapy. The patient is treated twice daily, six hours apart, for five days. After the tenth and final fraction is delivered, the balloon is deflated and removed.
Treatment is generally completed within 10 to 14 days after the patient is assessed for PBI. We always allow a two-week break from the completion of radiation therapy to the start of chemotherapy because of radiation recall concerns with some of the systemic agents, primarily doxorubicin.
Off-protocol use of the MammoSite®
The MammoSite® is easier for surgeons to use and patients to accept, but there’s concern that it’s being disseminated to the community before it’s fully tested in randomized trials. Many physicians argue that we cannot extrapolate the interstitial experience to the MammoSite® and that the interstitial experience itself is very limited with only five years of follow-up. Some worry that because it’s hyperfractionated radiation, we’ll encounter very late deterioration in cosmetic results not seen in the five-year data.
I favor enrolling patients in randomized trials; however, data from the current trials won’t be mature and analyzed for at least eight years after accrual is completed. I was involved in writing the ABS recommendations in which we stated that, with informed consent and in selected patients, it is reasonable to offer the MammoSite® off protocol. Most new concepts in medicine are not proven in Phase III trials before they’re used in clinical practice, as seen with sentinel node biopsy. I believe it’s more reasonable to give recommendations on the optimal use of this technique than to just oppose its use off protocol.
Select publications
 |
Dr Vicini is Chief of Oncology Services in Oncology Services Administration at William Beaumont Hospital in Royal Oak, Michigan. |
|
