| You are here: Home: BCU 7|2004: D Craig Allred, MD
D Craig Allred, MD |
EDITED COMMENTS |
 Estrogen receptor status and tamoxifen efficacy in patients with DCIS Estrogen receptor status and tamoxifen efficacy in patients with DCIS
NSABP-B-24 compared adjuvant tamoxifen to placebo in patients with DCIS. After four or five years of follow-up, the tamoxifen arm showed a 30 percent benefit, but we didn’t understand the relationship of this response rate to the tumor’s hormone receptor status. When the trial was initiated, assessing hormone receptors wasn’t required, but tumors were banked to conduct biological studies.
In a central lab, we later measured the estrogen and progesterone receptors by immunohistochemistry (IHC) on approximately 600 paraffin blocks distributed between the two arms of the study. The data convincingly demonstrated that the benefit from tamoxifen was entirely restricted to the ER-positive cohort, and there was no evidence of benefit in the ER-negative cohort. We know that approximately 25 percent of DCIS cases are truly ER-negative.
Approximately two thirds of the cases analyzed had hormone receptors previously evaluated in their community hospitals and, using the central lab as the standard, the community data demonstrated a 30 percent error rate — mostly false negatives (2.1). In the patients with ER-negative tumors, as defined by community labs, the relative risk for benefit from tamoxifen was approximately 0.5, which is unbelievable biologically.
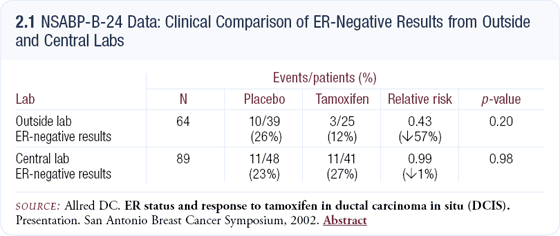
Assessing the same patients in the central lab, the relative risk was 0.99, indicating no benefit as we would expect. Clearly, the cohort of cases identified as ER-negative in the community was contaminated with false negatives. We can conclude from our data that tamoxifen does not reduce the recurrence rate in patients with truly ER-negative DCIS.
Contributing factors resulting in false negatives in estrogen receptor analysis
I consult on several hundred difficult cases each year. Many of these are sent for repeat estrogen receptor testing, and the conversion rate from negative to positive is 20 to 30 percent. The reasons for false negatives have been studied in detail in invasive cancer, and the same errors probably occur when assessing the estrogen receptor status in patients with DCIS.
The single biggest contributor to error is the antigen retrieval, which is an artsy part of the assay in which we try to reverse the cross-linking between the proteins caused by the initial formalin fixation. Another major problem is the antibody selected. Dozens of antibodies are available, and they are not equivalent in sensitivity and specificity.
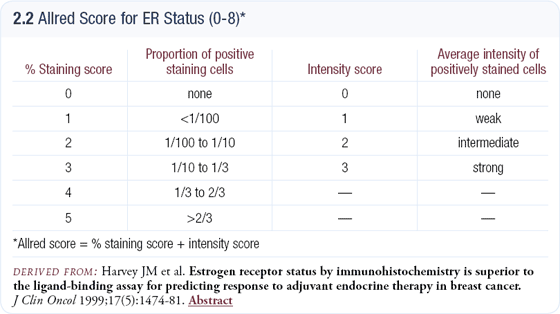
Setting the cut point for positivity too high is another significant error. It is usually set arbitrarily rather than based on clinical studies, and averages 10 or even 20 percent across the country. In invasive disease the cut point is much lower; almost so low that if it’s measurable, there’s probably a good chance the tumor will respond to hormonal therapy. The cut point we use — one percent — is based on clinical trials involving invasive breast cancer (2.2), but when applied to the NSABP-B-24 DCIS study, the results were very reasonable.
It’s worrisome that many community labs simply report the estrogen receptor status as positive or negative. A comprehensive report provides an impression as to the positivity or negativity of the specimen, a percent or a proportion of positive cells, and may footnote relevant clinical trials.
Biochemical ligand-binding versus IHC for estrogen receptors
Few clinical trials have used IHC to assess hormonal status. Those that have, such as NSABP-B-24, found significant problems with false-negative results, in terms of response, from outside laboratories. The international overview meta-analysis of adjuvant endocrine therapies is based almost entirely on biochemical ligand-binding testing. Similar to our experience with IHC today, the ligandbinding test initially suffered from a great deal of variability in results from different labs.
The cooperative groups, particularly the NSABP, moved quickly to require that hormonal profiles be assessed by labs with proven proficiency in ligand-binding assays before the patient could be enrolled in clinical trials. By the early 1980s, a relatively small number of qualified laboratories were performing the majority of tests.
Understanding the current variability problems with IHC assessment, reagent companies like Dako are working with my lab and others to develop a reliable kit-based test to measure hormone receptors and to provide labs with little experience or low test volume the capacity to perform high-quality tests. Unlike the HercepTest™, which was clinically available before it was properly validated, this kit will be based on clinical correlative studies.
Effect of phenotype on benefit in the ATAC trial
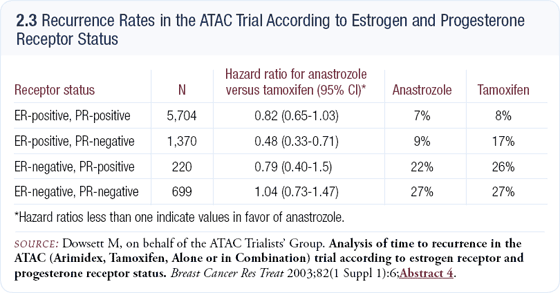
The ATAC data analyzing ER and PR phenotypes and benefit from therapy was fascinating. Compared to tamoxifen, anastrozole had approximately a 20 percent additional benefit in the ER-positive, PR-positive and ER-negative, PR-positive subsets. In the ER-negative, PR-negative phenotype, the relative risk was close to one, but surprisingly in the ER-positive, PR-negative subset, the relative risk was 0.48 (2.3).
We don’t know why the ER-positive, PR-negative phenotype behaves so differently, but Dowsett and Osborne have formulated a hypothesis that involves contrasting the effect of tamoxifen to that of anastrozole on the classical nuclear versus nonclassical membrane estrogen receptor pathways.
When the nuclear pathway is intact, estrogen activates the estrogen receptor, which induces the synthesis of the progesterone receptor; however, we can hypothesize that pathway is not functioning in ER-positive, PR-negative tumors. If the membrane pathway is activated, it can lead to the activation of growth factor receptors and induce cell growth.
Tamoxifen is an antagonist in the nuclear pathway (hypothetically, the nonfunctioning pathway in the ER-positive, PR-negative subset) and it’s an agonist in the membrane pathway, which may result in stimulating growth factors and tumor growth.
On the other hand, aromatase inhibitors reduce estrogen levels to nearly zero and are antagonists on both pathways. This may explain the striking additional benefit from anastrozole seen in the ER-positive, PR-negative subset, which is the phenotype for 20 percent of breast cancer patients.
The HER2 assays have not yet been performed in the ATAC trial, but some have speculated that the subset of patients with the ER-positive, PR-negative phenotype may also be HER2-positive. However, we’ve known for years that only 10 or 15 percent of HER2-positive tumors are ER-positive and, while most of those are PR-negative, I don’t believe that small subset could be entirely responsible for these intriguing results.
Quality control for HER2 testing
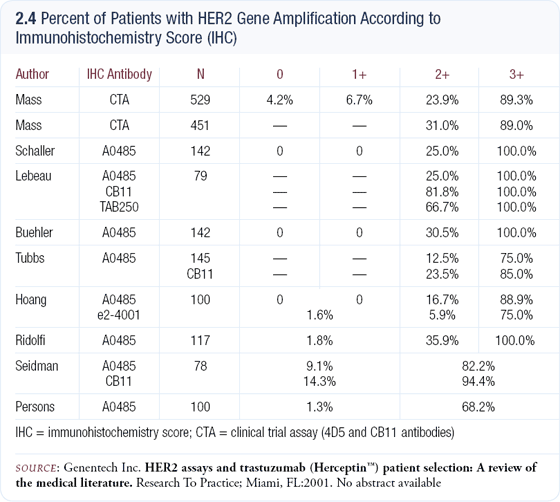
We still have substantial problems with HER2 testing in clinical practice. Most labs rely on IHC, but the quality varies tremendously (2.4). I don’t believe one should resort to FISH in every case for a number of reasons, including cost. If performed properly, IHC can provide an accurate answer 80 to 85 percent of the time. Using the HercepTest™ IHC criteria, I believe only the 15 or 20 percent of cases that are scored 2+ should be evaluated by FISH for resolution.
Another problem is that we don’t have a perfect algorithm for HER2 testing from which to make all decisions because the biology of HER2 is so complex. We know that approximately 10 percent of patients without gene amplification overexpress the protein, and it seems reasonable that those tumors would be as responsive to a targeted therapy, like trastuzumab, as tumors whose overexpression is the result of a HER2 gene amplicon.
A tremendous economic incentive exists to order FISH, which doesn’t necessarily translate to benefit. At the 2003 San Antonio Breast Cancer Symposium, two posters demonstrated a wide variation in 2+ positivity rates. These labs are either conducting or scoring the test differently. I suspect overinterpretation with IHC is more common than underinterpretation, possibly to justify resorting to FISH for resolution.
Select publications
 |
Dr Allred is a Professor of Pathology at the Baylor College of Medicine Breast Center in Houston, Texas. |
|
